- Dimedone
-
Dimedone 
 5,5-Dimethylcyclohexane-1,3-dioneOther namesCyclomethone,
5,5-Dimethylcyclohexane-1,3-dioneOther namesCyclomethone,
5,5-dimethyl-1,3-cyclohexanedione,
Dimethyldihydroresorcinol,
MethoneIdentifiers CAS number 126-81-8 
PubChem 31358 ChemSpider 29091 
Jmol-3D images Image 1 - O=C1CC(=O)CC(C)(C)C1
Properties Molecular formula C8H12O2 Molar mass 140.17968 Appearance Yellow crystals Melting point 147-150 °C (decomposes)
 (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Dimedone is a cyclic diketone used in organic chemistry to determine whether a compound contains an aldehyde group. Cyclohexanediones in general can be used as catalysts in the formation of transition-metal complexes. Other uses include applications in colourimetry, crystallography, luminescence and spectrophotometric analysis. It can also be used for chemistry involving organic compounds of low electrical resistance.
Contents
Synthesis
Dimedone is prepared from mesityl oxide and diethyl malonate[1].
Physical properties
Dimedone usually comes in the form of yellow crystals. It is stable under ambient conditions and soluble in water, as well as ethanol and methanol. It has a melting point range of 147 - 150 °C (420 - 423 K).
Tautomerism
Dimedone is in equilibrium with its tautomer in solution — in a 2:1 keto to enol ratio in chloroform[2].
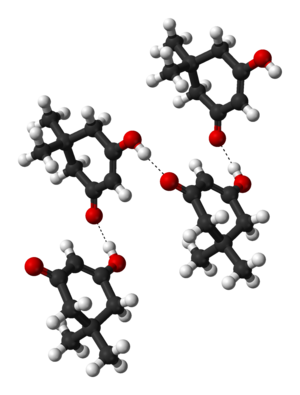
Crystalline dimedone contains chains of molecules, in the enol form, linked by hydrogen bonds[3]:
References
- ^ R. L. Shriner and H. R. Todd (1935). "5,5-dimethyl-1,3-cyclohexanedione". Organic Syntheses 15: 16. http://www.orgsyn.org/orgsyn/prep.asp?prep=cv2p0200.
- ^ Clayden, Jonathan; Greeves, Nick; Warren, Stuart; Wothers, Peter (2001). Organic Chemistry (1st ed.). Oxford University Press. pp. p. 532. ISBN 978-0-19-850346-0.
- ^ M. Bolte and M. Scholtyssik (October 1997). "Dimedone at 133K". Acta Cryst. C53 (10): IUC9700013. doi:10.1107/S0108270197099423.
Categories:- Ketones
Wikimedia Foundation. 2010.