- Methyl cinnamate
-
Methyl cinnamate[1][2] 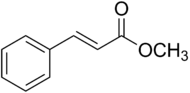
 Methyl (E)-3-Phenylprop-2-enoate
Methyl (E)-3-Phenylprop-2-enoateIdentifiers CAS number 1754-62-7 PubChem 637520 EC number 203-093-8 Jmol-3D images Image 1 - COC(=O)C=CC1=CC=CC=C1
Properties Molecular formula C10H10O2 Molar mass 162.185 g/mol Density 1.092 g/cm^3 Melting point 34-38 °C
Boiling point 261-262 °C
Solubility in water Insoluble Hazards S-phrases S22 S24/25 Flash point >230 °F  cinnamate (verify) (what is:
cinnamate (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references
Methyl cinnamate is the methyl ester of cinnamic acid and is a white or transparent solid with a strong, aromatic odor. It is found naturally in a variety of plants, including in fruits, like strawberry, and some culinary spices, such as Sichuan pepper and some varieties of basil.[3][4] Eucalyptus olida has the highest known concentrations of methyl cinnamate (98%) with a 2-6% fresh weight yield in the leaf and twigs.[5]Methyl cinnamate is used in the flavor and perfume industries. The flavor is fruity and strawberry-like; and the odor is sweet, balsamic with fruity odor, reminiscent of cinnamon and strawberry.[1]
It is known to attract males of various orchid bees, such as Aglae caerulea.[6]
List of plants that contain the chemical
- Eucalyptus olida 'Strawberry gum'
- Ocimum americanum cv.Purple Lovingly (Querendona Morada)
- Ocimum americanum cv. Purple Castle (Castilla Morada)
- Ocimum americanum cv. Purple Long-legged (Zancona morada)
- Ocimum americanum cv. Clove (Clavo)
- Ocimum basilicum cv. Sweet Castle (Dulce de Castilla)
- Ocimum basilicum cv. White Compact (Blanca compacta)
- Ocimum basilicum cv. large green leaves (Verde des horjas grandes)
- Ocimum micranthum cv. Cinnamon (Canela)
- Ocimum minimum cv. Little Virgin (Virgen pequena)
- Ocimum minimum cv. Purple Virgin (Virgen morada)
- Ocimum sp. cv. Purple ruffle (Crespa morada)
- Ocimum sp. cv. White Ruffle (Crespa blanca)
Toxicology & safety
Moderately toxic by ingestion. The oral LD50 for rats is 2610 mg/kg.[7] It is combustible as a liquid, and when heated to decomposition it emits acrid smoke and irritating fumes. [4]
Compendial status
- Food Chemicals Codex.[8]
References
- ^ a b Methyl cinnamate, at goodscents.com
- ^ Methyl cinnamate, at Sigma-Aldrich
- ^ Amparo Viña; Elizabeth Murillo, Essential oil composition from twelve varieties of basil (Ocimum spp) grown in Colombia, Journal of the Brazilian Chemical Society, vol.14 no.5 São Paulo Sept./Oct. 2003 http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0103-50532003000500008
- ^ a b Lookchem
- ^ Boland, D.J., Brophy, J.J., and A.P.N. House, Eucalyptus Leaf Oils, 1991, ISBN 0-909605-69-6
- ^ Williams, N.H.; Whitten, W.M. (1983). "Orchid floral fragrances and male euglossine bees: methods and advances in the last sesquidecade". Biol. Bull. 164 (3): 355–395. doi:10.2307/1541248.
- ^ Food and Cosmetics Toxicology. No. 13 (1975),p681.
- ^ Therapeutic Goods Administration (1999). "Approved Terminology for Medicines". http://www.tga.gov.au/docs/pdf/aan/aan.pdf. Retrieved 29 June 2009.
See also
Categories:- Carboxylate esters
- Methyl esters
- Flavors
- Alkenes
Wikimedia Foundation. 2010.

