- Rossmann fold
-
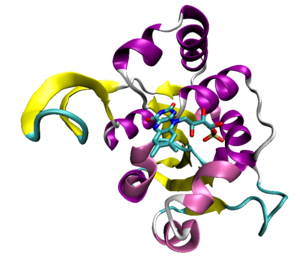 An example of the Rossmann fold, a structural domain of a decarboxylase protein from the bacterium Staphylococcus epidermidis (PDB ID 1G5Q) with the bound flavin mononucleotide cofactor shown.
An example of the Rossmann fold, a structural domain of a decarboxylase protein from the bacterium Staphylococcus epidermidis (PDB ID 1G5Q) with the bound flavin mononucleotide cofactor shown.
The Rossmann fold is a protein structural motif found in proteins that bind nucleotides, especially the cofactor NAD. The structure with two repeats is composed of six parallel beta strands linked to two pairs of alpha helices in the topological order beta-alpha-beta-alpha-beta. Because each Rossmann fold can bind one nucleotide, binding domains for dinucleotides such as NAD consist of two paired Rossmann folds that each bind one nucleotide moiety of the cofactor molecule. Single Rossmann folds can bind mononucleotides such as the cofactor FMN.
The motif is named for Michael Rossmann, who first pointed out that this is a frequently occurring motif in nucleotide binding proteins, such as dehydrogenases.[1]
References
- ^ Rao S, Rossmann M (1973). "Comparison of super-secondary structures in proteins". J Mol Biol 76 (2): 241–56. doi:10.1016/0022-2836(73)90388-4. PMID 4737475.
General All-α folds: All-β folds: α/β folds: TIM barrel · Leucine-rich repeat · Flavodoxin fold · Rossmann fold · Thioredoxin fold · Trefoil knot foldα+β folds: Irregular folds: ←Secondary structure
This protein-related article is a stub. You can help Wikipedia by expanding it.
