- Dirlotapide
-
Dirlotapide 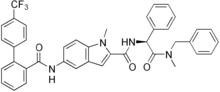
Systematic (IUPAC) name 1-Methyl-N-[(1S)-2-(methyl-(phenylmethyl)amino)-2-oxo-1-phenylethyl]-5-[ [oxo-[2-[4-(trifluoromethyl)phenyl]phenyl]methyl]amino]-2-indolecarboxamide Clinical data AHFS/Drugs.com International Drug Names Pregnancy cat. ? Legal status ? Identifiers CAS number 481658-94-0 ATCvet code QA08AB91 PubChem CID 9917862 ChemSpider 8093509 
UNII 578H0RMP25 
KEGG D03867 
ChEMBL CHEMBL410414 
Chemical data Formula C40H33F3N4O3 Mol. mass 674.71 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Dirlotapide is a drug used to treat obesity in dogs. It is manufactured by Pfizer and marketed as Slentrol.
It works as a selective microsomal triglyceride transfer protein inhibitor. This blocks the assembly and release of lipoproteins into the bloodstream, thereby reducing fat absorption. It also elicits a satiety signal from lipid-filled cells lining the intestine.
It is supplied as an oral solution. It is not intended for use in humans, cats, or parrots.
On January 5 2007, the U.S. Food and Drug Administration (FDA) approved Slentrol, the first time the FDA has approved a drug for obese dogs.[1]
References
- ^ "FDA approves 1st drug for obese dogs". Yahoo. Archived from the original on January 8, 2007. http://web.archive.org/web/20070108052816/http://news.yahoo.com/s/ap/20070106/ap_on_he_me/doggie_diet_drug. Retrieved 2007-01-06.
External links

This veterinary medicine–related article is a stub. You can help Wikipedia by expanding it.
