- Cholestane
-
Cholestane 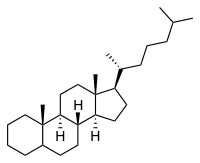
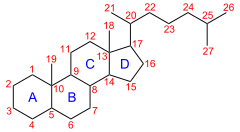
IUPAC numbering [1](8R,9S,10S,13R,14S,17R)-10,13-Dimethyl-17-[(2R)-6-methylheptan-2-yl]-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthreneIdentifiers CAS number 481-21-0 PubChem 6857534 ChemSpider 5256870 
ChEBI CHEBI:35516 
Jmol-3D images Image 1
Image 2- C[C@H](CCCC(C)C)[C@H]1CC[C@@H]2[C@@]1(CC[C@H]3[C@H]2CCC4[C@@]3(CCCC4)C)C
C41CCCC[C@@]1([C@@H]3[C@H]([C@@H]2CC[C@@H]([C@@]2(C)CC3)[C@H](C)CCCC(C)C)CC4)C
- InChI=1S/C27H48/c1-19(2)9-8-10-20(3)23-14-15-24-22-13-12-21-11-6-7-17-26(21,4)25(22)16-18-27(23,24)5/h19-25H,6-18H2,1-5H3/t20-,21?,22+,23-,24+,25+,26+,27-/m1/s1

Key: XIIAYQZJNBULGD-LDHZKLTISA-N
InChI=1/C27H48/c1-19(2)9-8-10-20(3)23-14-15-24-22-13-12-21-11-6-7-17-26(21,4)25(22)16-18-27(23,24)5/h19-25H,6-18H2,1-5H3/t20-,21?,22+,23-,24+,25+,26+,27-/m1/s1
Key: XIIAYQZJNBULGD-LDHZKLTIBN
Properties Molecular formula C27H48 Molar mass 372.67 g mol−1  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Cholestane is a saturated 27-carbon steroid precursor which serves as the basis for many organic molecules.
Derivatives of cholestane
Derivatives are classified in two families:
- Sterols (with an alcohol group)
- Cholestenes (with a double bond)
Some steroids, such as cholesterol, are both a sterol and a cholestene.
External links
Categories:- Steroids
- Hydrocarbons
- Ester stubs
- C[C@H](CCCC(C)C)[C@H]1CC[C@@H]2[C@@]1(CC[C@H]3[C@H]2CCC4[C@@]3(CCCC4)C)C
Wikimedia Foundation. 2010.
