- Schwartz's reagent
-
Schwartz's reagent 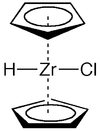
 chloridobis(η5-cyclopentadienyl)hydridozirconiumOther namesCp2ZrClH, zirconocene hydride
chloridobis(η5-cyclopentadienyl)hydridozirconiumOther namesCp2ZrClH, zirconocene hydrideIdentifiers CAS number 37342-97-5 Properties Molecular formula C10H11ClZr Molar mass 257.87 g/mol Appearance White solid  reagent (verify) (what is:
reagent (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Schwartz's reagent is the common name for the chemical compound with the formula (C5H5)2ZrHCl, sometimes described zirconocene hydrochloride or zirconocene chloride hydride and is named after Jeffrey Schwartz, who is currently a professor in Chemistry at Princeton University. This metallocene is used in organic synthesis for various transformations of alkenes and alkynes.[1][2][3]
Contents
Hydrozirconation
Schwartz's reagent reacts with alkenes and alkynes via the process called hydrozirconation which formally results in the addition of the Zr-H bond across the C=C or C≡C bond. The selectivity of the hydrozirconation of alkynes has been studied in detail.[4][5] Generally, the addition of the Zr-H proceeds the syn-addition. The rate of addition to unsaturated carbon-carbon bonds is terminal alkyne > terminal alkene ~ internal alkyne > disubstituted alkene [6] Acyl complexes can be generated by insertion of CO into the C-Zr bond resulting from hydrozirconation.[7] Upon alkene insertion into the zirconium hydride bond, the resulting zirconium alkyl undergoes facile rearrangement to the terminal alkyl and therefore only terminal acyl compounds can be synthesized in this way. The rearrangement most likely proceeds via β-hydride elimination followed by reinsertion.
Preparation
The complex was first prepared by Wailes and Weigold.[8] It can be purchased or readily prepared by reduction of zirconocene dichloride with lithium aluminium hydride:
- (C5H5)2ZrCl2 + 1/4 LiAlH4 → (C5H5)2ZrHCl + 1/4 "LiAlCl4"
In practice this reaction also makes (C5H5)2ZrH2, which is treated with methylene chloride to give the mixed hydride chloride.[9] An alternative procedure that generated Schwartz's Reagent from dihydride has also been reported.[10]
References
- ^ D. W. Hart and J. Schwartz (1974). "Hydrozirconation. Organic Synthesis via Organozirconium Intermediates. Synthesis and Rearrangement of Alkylzirconium(IV) Complexes and Their Reaction with Electrophiles". J. Am. Chem. Soc. 96 (26): 8115–8116. doi:10.1021/ja00833a048.
- ^ J. Schwartz, and J. A. Labinger (2003). "Hydrozirconation: A New Transition Metal Reagent for Organic Synthesis". Angew. Chem. Int. Ed. 15 (6): 330–340. doi:10.1002/anie.197603331.
- ^ Donald W. Hart, Thomas F. Blackburn, Jeffrey Schwartz (1975). "Hydrozirconation. III. Stereospecific and regioselective functionalization of alkylacetylenes via vinylzirconium(IV) intermediates". J. Am. Chem. Soc. 97 (3): 679–680. doi:10.1021/ja00836a056.
- ^ R. C. Sun, M. Okabe, D. L. Coffen, and J. Schwartz (1998), "Conjugate Addition of a Vinylzirconium Reagent: 3-(1-Octene-1-yl)cyclopentanone", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv9p0640; Coll. Vol. 9: 640
- ^ Panek, J. S.; Hu, T. (1997). "Stereo- and Regiocontrolled Synthesis of Branched Trisubstituted Conjugated Dienes by Palladium(0)-Catalyzed Cross-Coupling Reaction". J. Org. Chem. 62 (15): 4912–4913. doi:10.1021/jo970647a.
- ^ Peter Wipf and Heike Jahn (1996). "Synthetic applications of organochlorozirconocene complexes". Tetrahedron 52 (40): 12853–12910. doi:10.1016/0040-4020(96)00754-5.
- ^ Christopher A. Bertelo, Jeffrey Schwartz (1975). "Hydrozirconation. II. Oxidative homologation of olefins via carbon monoxide insertion into the carbon-zirconium bond". J. Am. Chem. Soc. 97 (1): 228–230. doi:10.1021/ja00834a061.
- ^ P. C. Wailes and H. Weigold (1970). "Hydrido complexes of zirconium I. Preparation". Journal of Organometallic Chemistry 24 (2): 405–411. doi:10.1016/S0022-328X(00)80281-8.
- ^ S. L. Buchwald, S. J. LaMaire, R. B. Nielsen, B. T. Watson, and S. M. King, "Schwartz's Reagent", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV9P0162; Coll. Vol. 9: 162
- ^ Peter Wipf, Hidenori Takahashi, and Nian Zhuang (1998). "Kinetic vs. thermodynamic control in hydrozirconation reactions". Pure Appl. Chem. 70 (5): 1077–1082. doi:10.1351/pac199870051077. http://www.iupac.org/publications/pac/1998/pdf/7005x1077.pdf.
External links
Categories:- Metallocenes
- Organozirconium compounds
- Cyclopentadienyl complexes
- Reagents for organic chemistry
Wikimedia Foundation. 2010.
