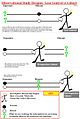
- Cohort study
-
For other senses of this word, see cohort (disambiguation).
A cohort study or panel study is a form of longitudinal study (a type of observational study) used in medicine, social science, actuarial science, and ecology. It is an analysis of risk factors and follows a group of people who do not have the disease, and uses correlations to determine the absolute risk of subject contraction. It is one type of clinical study design and should be compared with a cross-sectional study. Cohort studies are largely about the life histories of segments of populations, and the individual people who constitute these segments.[1][2]
A cohort is a group of people who share a common characteristic or experience within a defined period (e.g., are born, are exposed to a drug or vaccine or pollutant, or undergo a certain medical procedure). Thus a group of people who were born on a day or in a particular period, say 1948, form a birth cohort. The comparison group may be the general population from which the cohort is drawn, or it may be another cohort of persons thought to have had little or no exposure to the substance under investigation, but otherwise similar. Alternatively, subgroups within the cohort may be compared with each other.
Randomized controlled trials, or RCTs are a superior methodology in the hierarchy of evidence in therapy, because they limit the potential for any biases by randomly assigning one patient pool to an intervention and another patient pool to non-intervention (or placebo). This minimizes the chance that the incidence of confounding (particularly unknown confounding) variables will differ between the two groups. However, it is important to note that RCTs may not be suitable in all cases and other methodologies would be much more suitable to investigate the study's objective
Cohort studies can either be conducted prospectively, or retrospectively from archived records.[3]
Contents
Application
In medicine, a cohort study is often undertaken to obtain evidence to try to refute the existence of a suspected association between cause and effect; failure to refute a hypothesis strengthens confidence in it. Crucially, the cohort is identified before the appearance of the disease under investigation. The study groups follow a group of people who do not have the disease for a period of time and see who develops the disease (new incidence). The cohort cannot therefore be defined as a group of people who already have the disease. Prospective (longitudinal) cohort studies between exposure and disease strongly aid in studying causal associations, though distinguishing true causality usually requires further corroboration from further experimental trials.
The advantage of prospective cohort study data is that it can help determine risk factors for contracting a new disease because it is a longitudinal observation of the individual through time, and the collection of data at regular intervals, so recall error is reduced. However, cohort studies are expensive to conduct, are sensitive to attrition and take a long follow-up time to generate useful data. Nevertheless, the results that are obtained from long-term cohort studies are of substantially superior quality to retrospective/cross-sectional studies, and cohort studies are considered the gold standard in observational epidemiology. Moreover, cohort studies are informative for efficiently studying a wide-range of exposure-disease associations.
Some cohort studies track groups of children from their birth, and record a wide range of information (exposures) about them. The value of a cohort study depends on the researchers' capacity to stay in touch with all members of the cohort. Some of these studies have continued for decades.
Examples
An example of an epidemiological question that can be answered by the use of a cohort study is: does exposure to X (say, smoking) associate with outcome Y (say, lung cancer)? Such a study would recruit a group of smokers and a group of non-smokers (the unexposed group) and follow them for a set period of time and note differences in the incidence of lung cancer between the groups at the end of this time. The groups are matched in terms of many other variables such as economic status and other health status so that the variable being assessed, the independent variable (in this case, smoking) can be isolated as the cause of the dependent variable (in this case, lung cancer). In this example, a statistically significant increase in the incidence of lung cancer in the smoking group as compared to the non-smoking group is evidence in favor of the hypothesis. However, rare outcomes, such as lung cancer, are generally not studied with the use of a cohort study, but are rather studied with the use of a case-control study.
Shorter term studies are commonly used in medical research as a form of clinical trial, or means to test a particular hypothesis of clinical importance. Such studies typically follow two groups of patients for a period of time and compare an endpoint or outcome measure between the two groups.
Randomized controlled trials, or RCTs are a superior methodology in the hierarchy of evidence, because they limit the potential for bias by randomly assigning one patient pool to an intervention and another patient pool to non-intervention (or placebo). This minimizes the chance that the incidence of confounding variables will differ between the two groups.
Nevertheless, it is sometimes not practical or ethical to perform RCTs to answer a clinical question. To take our example, if we already had reasonable evidence that smoking causes lung cancer then persuading a pool of non-smokers to take up smoking in order to test this hypothesis would generally be considered quite unethical.
Two examples of cohort studies that have been going on for more than 50 years are the Framingham Heart Study and the National Child Development Study (NCDS), the most widely-researched of the British birth cohort studies.
Key findings of NCDS and a detailed profile of the study appear in the International Journal of Epidemiology.[4]
International Journal of Epidemiology[5] comparison of two Cohorts, Millennium Cohort Study (United States) and the The King’s Cohort (United Kingdom)
The largest cohort study in women is the Nurses' Health Study. Started in 1976, it is tracking over 120,000 nurses and has been analyzed for many different conditions and outcomes.
The largest cohort study in Africa is the Birth to Twenty Study which began in 1990 and tracks a cohort of over 3,000 children born in the weeks following Nelson Mandela's release from prison.
Other famous examples are the Grant Study tracking a number of Harvard graduates from ca. 1950.77, and the Whitehall Study tracking 10,308 British civil servants.
Variations
Retrospective cohort
A "prospective cohort" defines the groups before the study is done, while a "retrospective cohort" defines the grouping after the data is collected. Examples of a retrospective cohort are Long-Term Mortality after Gastric Bypass Surgery.[6] and The Lothian Birth Cohort Studies.[7]
Nested case-control study
An example of a nested case-control study is Inflammatory markers and the risk of coronary heart disease in men and women, which was a case control analyses extracted from the Framingham Heart Study cohort.[8]
Household panel survey
Household panel surveys are an important sub-type of cohort study. These draw representative samples of households and survey them, following all individuals through time on a usually annual basis. Examples include the US Panel Study on Income Dynamics (since 1968), the German Socio-Economic Panel (since 1984), the British Household Panel Survey (since 1991), the Household, Income and Labour Dynamics in Australia Survey (since 2001) and the European Community Household Panel (1994–2001).
See also
References
- ^ http://www.socialresearchmethods.net/tutorial/Cho2/cohort.html
- ^ Porta M (editor). A dictionary of epidemiology. 5th. edition. New York: Oxford University Press, 2008. [1]
- ^ http://www.ehib.org/faq.jsp?faq_key=37
- ^ Power C and Elliott J (2006). "Cohort profile: 1958 British Cohort Study". International Journal of Epidemiology 35 (1): 34–41. doi:10.1093/ije/dyi183. PMID 16155052.
- ^ Richard J Pinder, Neil Greenberg, Edward J Boyko, Gary D Gackstetter, Tomoko I Hooper, Dominic Murphy, Margaret AK Ryan, Besa Smith, Tyler C Smith, Timothy S Wells and Simon Wessely (2011-6-29). "Profile of two cohorts: UK and US prospective studies of military health". International Journal of Epidemiology (Oxford Journals). doi:10.1093/ije/dyr096. http://ije.oxfordjournals.org/content/early/2011/06/29/ije.dyr096.full.
- ^ Adams TD, Gress RE, Smith SC et al. (2007). "Long-term mortality after gastric bypass surgery". N. Engl. J. Med. 357 (8): 753–61. doi:10.1056/NEJMoa066603. PMID 17715409.
- ^ "The Lothian Birth Cohort Studies". University of Edinburgh. http://www.psy.ed.ac.uk/research/lbc/LBC.html. Retrieved 8 May 2011.
- ^ Pai JK, Pischon T, Ma J et al. (2004). "Inflammatory markers and the risk of coronary heart disease in men and women". N. Engl. J. Med. 351 (25): 2599–610. doi:10.1056/NEJMoa040967. PMID 15602020.
External links
- Prospective cohorts
- Retrospective cohorts
- Study Design Tutorial Cornell University College of Veterinary Medicine
- Birth cohort study timelines (ESDS Longitudinal)
- Centre for Longitudinal Studies
Biomedical research: Clinical study design / Design of experiments Overview Controlled study
(EBM I to II-1; A to B)Observational study
(EBM II-2 to II-3; B to C)Cross-sectional study vs. Longitudinal study, Ecological study
Cohort study (Retrospective cohort study, Prospective cohort study)
Case-control study (Nested case-control study)
Case series · Case study / Case reportEpidemiology/
methodsoccurrence: Incidence (Cumulative incidence) · Prevalence (Point prevalence, Period prevalence)
association: absolute (Absolute risk reduction, Attributable risk, Attributable risk percent) · relative (Relative risk, Odds ratio, Hazard ratio)
other: Virulence · Infectivity · Mortality rate · Morbidity · Case fatality · Specificity and sensitivity · Likelihood-ratios · Pre/post-test probabilityTrial/test types Analysis of clinical trials Risk–benefit analysis
Interpretation of results Category · Glossary · List of topics Categories:- Cohort studies
- Cohort study methods
Wikimedia Foundation. 2010.