- Iron oxide
-
Iron oxides are chemical compounds composed of iron and oxygen. All together, there are three hundred known iron oxides and oxyhydroxides.[1]
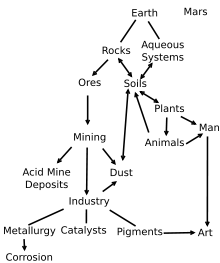
Iron oxides and oxide-hydroxides are widespread in nature, play an important role in many geological and biological processes, and are widely utilized by humans, e.g., as iron ores, pigments, catalysts, in thermite (see the diagram). Common rust is a form of iron(III) oxide.
Contents
Oxides
- iron(II) oxide, wüstite (FeO)
- iron(II,III) oxide, magnetite (Fe3O4)
- iron(III) oxide (Fe2O3)
- alpha phase, hematite (α-Fe2O3)
- beta phase, (β-Fe2O3)
- gamma phase, maghemite (γ-Fe2O3)
- epsilon phase, (ε-Fe2O3)
Hydroxides
- iron(II) hydroxide (Fe(OH)2)
- iron(III) hydroxide (Fe(OH)3), (bernalite)
Oxide/hydroxides
Main article: iron(III) oxide-hydroxide- goethite (α-FeOOH),
- akaganéite (β-FeOOH),
- lepidocrocite (γ-FeOOH),
- feroxyhyte (δ-FeOOH),
- ferrihydrite (Fe5HO8·4H2O approx.), or 5Fe2O3•9H2O, better recast as FeOOH•0.4H2O
- high-pressure FeOOH
- schwertmannite (ideally Fe8O8(OH)6(SO)·nH2O or Fe3+16O16(OH,SO4)12-13·10-12H2O)[2]
- green rusts (FeIIIxFeIIy(OH)3x+2y-z(A-)z; where A- is Cl- or 0.5SO42-)
See also
- Limonite
- Iron oxide nanoparticles
References
- ^ Cornell, RM; Schwertmann, U (2003). The iron oxides: structure, properties, reactions, occurrences and uses. Wiley VCH. ISBN 3-527-30274-3.
- ^ http://www.mindat.org/min-7281.html Mindat
External links
Categories:- Iron compounds
- Oxides
- Iron oxide pigments
Wikimedia Foundation. 2010.