- Chlorotoluene
-
Chlorotoluene can refer to any of four isomeric chemical compounds. Three isomers (ortho-chlorotoluene, meta-chlorotoluene, and para-chlorotoluene) consisist of a disubsituted benzene ring with one chlorine atom and one methyl group. The fourth isomer, alpha-chlorotoluene (benzyl chloride), consists of toluene with chlorine substitution on the methyl group.
Chemical properties
There are three ring-substituted chlorotoluenes, as well as benzyl chloride, which has a chlorine substituted for one of the hydrogens of toluene's the methyl group. These isomers differ in the location of the chlorine, but have the same chemical formula. All have very similar boiling points, although p-chlorotoluene has a much higher melting point due a more tightly-packed crystal structure.
Chlorotoluene Isomers General Common name o-chlorotoluene m-chlorotoluene p-chlorotoluene Benzyl chloride Structure 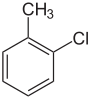
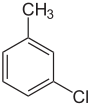


Systematic name 1-chloro-2-methylbenzene 1-chloro-3-methylbenzene 1-chloro-4-methylbenzene chloromethylbenzene Molecular formula C7H7Cl (C6H4ClCH3) Molar mass 126.586 g/mol Appearance clear, colorless liquid CAS number [95-49-8] [108-41-8] [106-43-4] [100-44-7] Properties Density and phase 1.073 g/mL, liquid 1.072 g/mL, liquid 1.069 g/mL, liquid 1.100 g/mL, liquid Solubility in water practically insoluble Other solubilities Soluble in non-polar solvents such as aromatic hydrocarbons Melting point −35 °C (-31 °F; 238 K) -47 °C (-52.6 °F; 226 K) 7 °C (44.6 °F; 280 K) -39 °C (-38.2 °F; 234 K) Boiling point 159 °C (318.2 °F; 432 K) 162 °C (323.6 °F; 435 K) 162 °C (323.6 °F; 435 K) 179 °C (354.2 °F; 452 K) Categories:- Organochlorides
Wikimedia Foundation. 2010.
