- Melilite
-
Melilite 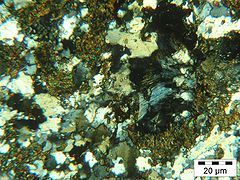
Orthite and melilite (blue) with quartz, from a thin section in crossed polarized light.General Category Sorosilicates Chemical formula (Ca,Na)2(Al,Mg,Fe2+)[(Al,Si)SiO7] Strunz classification 09.BB.** Identification Color Yellowish, greenish brown Crystal habit Massive - granular Crystal system Tetragonal Cleavage Distinct on {001}, weak on {110} Fracture Uneven Mohs scale hardness 5 - 5.5 Luster Vitreous - greasy Streak white Diaphaneity Translucent Specific gravity 2.9- 3.0 Optical properties Uniaxial (-) Refractive index nω = 1.632 - 1.669 nε = 1.626 - 1.658 Birefringence δ = 0.006 - 0.011 References [1][2] Melilite refers to a mineral of the melilite group. Minerals of the group are solid solutions of several endmembers, the most important of which are gehlenite and åkermanite. A generalized formula for common melilite is (Ca,Na)2(Al,Mg,Fe2+)[(Al,Si)SiO7]. Discovered in 1793 near Rome, it has a yellowish, greenish brown color. The name derives from the Greek words meli (μέλι) "honey" and lithos (λίθους) "stone".
Minerals of the melilite group are sorosilicates. They have the same basic structure, of general formula A2B(T2O7). The melilite structure consist of pairs of fused TO4, where T may be Si, Al, B, in bow-tie form. Sharing one corner, the formula of the pair is T2O7. These bow-ties are linked together into sheets by the B cations. The sheets are held together by the A cations, most commonly calcium and sodium. Aluminium may sit on either the T or the B site.
Minerals with the melilite structure may show a cleavage parallel to the (001) crystallographic directions and may show weaker cleavage perpendicular to this, in the {110} directions. Melilite is tetragonal.
The important endmembers of common melilite are åkermanite Ca2Mg(Si2O7) and gehlenite Ca2Al[AlSiO7]. Many melilites also contain appreciable iron and sodium.
Some other compositions with the melilite structure include: alumoåkermanite (Ca,Na)2(Al,Mg,Fe2+)(Si2O7), okayamalite Ca2B[BSiO7], gugiaite Ca2Be[Si2O7], hardystonite Ca2Zn[Si2O7], barylite BaBe2[Si2O7], andremeyerite BaFe2+2[Si2O7]. Some structures formed by replacing one oxygen by F or OH: leucophanite (Ca,Na)2(Be,Al)[Si2O6(F,OH)], jeffreyite (Ca,Na)2(Be,Al)[Si2O6(O,OH)], and meliphanite (Ca,Na)2(Be,Al)[Si2O6(OH,F)]
Contents
Occurrences
Melilite with compositions dominated by the endmembers akermanite and gehlenite is widely distributed but uncommon. It occurs in metamorphic and igneous rocks and in meteorites.
Typical metamorphic occurrences are in high-temperature metamorphosed impure limestones. For instance, melilite occurs in some high-temperature skarns.
Melilite also occurs in unusual silica-undersaturated igneous rocks. Some of these rocks appear to have formed by reaction of magmas with limestone. Other igneous rocks containing melilite crystallize from magma derived from the Earth's mantle and apparently uncontaminated by the Earth's crust. The presence of melilite is an essential constituent in some rare igneous rocks, such as olivine melilitite. Extremely rare igneous rocks contain as much as 70% melilite, together with minerals such as pyroxene and perovskite.
Melilite is a constituent of some calcium- and aluminium-rich inclusions (CAIs) in chondritic meteorites[3]. Isotope ratios of magnesium and some other elements in these inclusions are of great importance in deducing processes that formed our solar system.
See also
References
- ^ http://webmineral.com/data/Melilite.shtml Webmineral
- ^ http://www.mindat.org/min-2635.html Mindat
- ^ Rubin, Alan E. (March 1997). "Mineralogy of meteorite groups". Meteoritics & Planetary Science 32 (2): 231–247. Bibcode 1997M&PS...32..231R. doi:10.1111/j.1945-5100.1997.tb01262.x.
- Deer, W.A., Howie, R.A., and Zussman, J. (1986) Disilicates and Ring Silicates, Rock-forming minerals 1B, 2nd Ed., New York : John Wiley & Sons, ISBN 0-582-46521-4
External links
Categories:- Calcium minerals
- Aluminium minerals
- Iron minerals
- Magnesium minerals
- Sorosilicates
- Tetragonal minerals
Wikimedia Foundation. 2010.