- Dynamic covalent chemistry
-
In supramolecular chemistry, dynamic covalent chemistry is a strategy that aims at synthesizing large complex molecules. In it a reversible reaction is under thermodynamic reaction control and a specific reaction product out of many is captured.[1] Because all the components in the reaction mixture are able to equilibrate quickly, (according to its advocates) some degree of error checking and proof reading is enabled. The concept of dynamic covalent chemistry was demonstrated in the development of specific molecular Borromean rings.
The underlying idea is that rapid equilibration allows the coexistence of a huge variety of different species among which one can select molecules with desired chemical, pharmaceutical and biological properties. For instance, the addition of a proper template will shift the equilibrium toward the component that forms the complex of higher stability (thermodynamic template effect). After the new equilibrium is established, the researcher modifies the reaction conditions so as to stop equilibration. The optimal binder for the template is then extracted from the reactional mixture by the usual laboratory procedures.
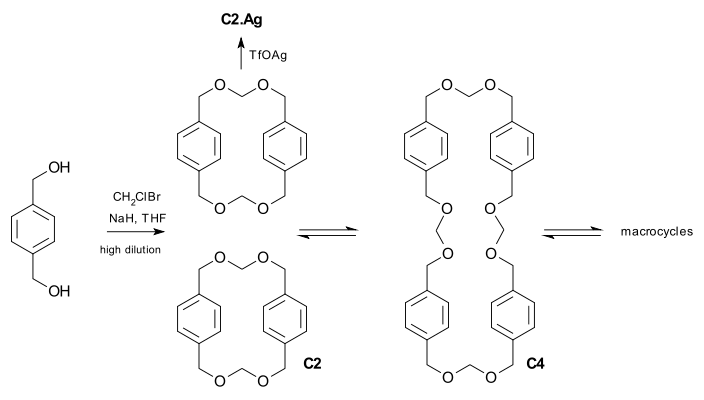
The concept is demonstrated in a minor but illustrative example involving polyacetal macrocycles.[2] The cyclophane C2 can be prepared by the irreversible highly diluted reaction of a diol with chlorobromomethane in the presence of sodium hydride. The dimer however is part of series of equilibria between polyacetal macrocycles of different size brought about by acid catalyzed (triflic acid) transacetalization.[3] Regardless of the starting material, C2, C4 or a high molar mass product, the equilibrium will eventually produce an identical product distribution. In this system it is also possible to amplify the presence of C2 in the mixture when the catalyst is silver triflate because the silver ion fits ideally and irreversibly in its cavity.
See also
- Organic chemistry
- Supramolecular chemistry
- Template effect
- Boronic acids in supramolecular chemistry: Saccharide recognition
References
- ^ Rowan SJ, Cantrill SJ, Cousins GR, Sanders JK, Stoddart JF (March 2002). "Dynamic covalent chemistry". Angew. Chem. Int. Ed. Engl. 41 (6): 898–952. doi:10.1002/1521-3773(20020315)41:6<898::AID-ANIE898>3.0.CO;2-E. PMID 12491278.
- ^ Cacciapaglia R, Di Stefano S, Mandolini L (October 2005). "Metathesis reaction of formaldehyde acetals: an easy entry into the dynamic covalent chemistry of cyclophane formation". J. Am. Chem. Soc. 127 (39): 13666–71. doi:10.1021/ja054362o10.1021/ja054362o. PMID 16190732.
- ^ This particular type of transacetalization goes by the name of formal metathesis because it is reminiscent of olefin metathesis but then with formaldehyde.
Categories:
Wikimedia Foundation. 2010.