- Covalent organic framework
-
The design and synthesis of crystalline extended organic structures in which the building blocks are linked by strong covalent bonds are core concepts of covalent organic frameworks (COFs). COFs are porous, and crystalline, and made entirely from light elements (H, B, C, N, and O) that are known to form strong covalent bonds in well-established and useful materials such as diamond, graphite, and boron nitride.
The successful realization of COF materials through molecular building blocks would provide covalent frameworks that could be functionalized into lightweight materials optimized for gas storage, photonic, and catalytic applications.[1]
Contents
Structure
Porous crystalline solids consists of secondary building units (SBUs) which assemble to form a periodic and porous framework.
An almost infinte numbers of frameworks can be formed through various SBU combinations leading to unique material properties for applications in separations, storage, and heterogeneous catalysis.[2]
Porous crystalline solids can be used to describe materials such as Zeolite, Metal-organic frameworks (MOFs), and Covalent Organic Frameworks (COFs).
Zeolites are microporous, aluminosilicate minerals commonly used as commercial adsorbents.
MOFs are a class of porous polymeric material, consisting of metal ions linked together by organic bridging ligands and are a new development on the interface between molecular coordination chemistry and materials science.[3]
COFs are another class of porous polymeric materials, consisting of porous, crystalline, covalent bonds that usually have rigid structures, exceptional thermal stabilities (to temperatures up to 600°C), and low densities. They exhibit permanent porosity with specific surface areas surpassing those of well-known zeolites and porous silicates.[1]
Secondary building units
The term ‘secondary building unit’ has been used for some time to describe conceptual fragments which can be compared as bricks used to build a house of zeolites; in the context of this page it refers to the geometry of the units defined by the points of extension.[4]
Reticular synthesis
Although the synthesis of new materials has long been recognized as the most essential element in advancing technology, it generally remains more of an art than a science—in that the discovery of new compounds has mostly been serendipitous, using methods referred to by critics as ‘shake and bake’, ‘mix and wait’ and ‘heat and beat’. It was caused by that the starting entities do not maintain their structure during the reaction, leading to poor correlation between reactants and products. However, the design of an extended network that will maintain their structural integrity throughout the construction process can be realized by starting with well-defined and rigid molecular building blocks.
In essence, reticular synthesis can be described as the process of assembling judiciously designed rigid secondary building units into predetermined ordered structures (networks), which are held together by strong bonding. It is different from retrosynthesis of organic compounds, because the structural integrity and rigidity of the building blocks in reticular synthesis remain unaltered throughout the construction process—an important aspect that could help to fully realize the benefits of design in crystalline solid-state frameworks. Similarly, reticular synthesis should be distinguished from supramolecular assembly, because in the former, building blocks are linked by strong bonds throughout the crystal.[4]
Applications
Hydrogen storage
Omar M. Yaghi and William A. Goddard III reported COFs as exceptional hydrogen storage materials. They predicted the highest excess H2 uptakes at 77 K are 10.0 wt % at 80 bar for COF-105, and 10.0 wt % at 100 bar for COF-108, which have higher surface area and free volume, by grand canonical Monte Carlo (GCMC) simulations as a function of temperature and pressure. This is the highest value reported for associative H2 storage of any material. Thus 3-D COFs are most promising new candidates in the quest for practical H2 storage materials.[5]
Optical properties
The ultimate highly ordered π-conjugation TP-COF, consisting of pyrene and triphenylene functionalities alternately linked in a mesoporous hexagonal skeleton, is highly luminescent, harvests a wide wavelength range of photons, and allows energy transfer and migration. Furthermore, TP-COF is electrically conductive and capable of repetitive on–off current switching at room temperature.[6]
Porosity/surface-area effects
Most studies to date have focused on the development of synthetic methodologies with the aim of maximizing pore size and surface area for gas storage. That means the functions of COFs have not yet been well explored, but COFs can be used as catalyst, or gas separation etc.[1]
History
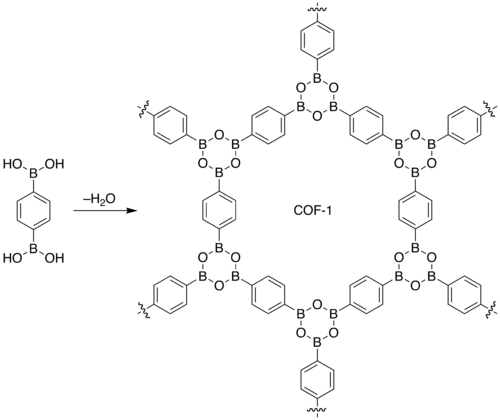
Omar M. Yaghi, a professor at the University of California, Los Angeles and Adrien P Cote published the first paper of COF.[1] They reported the design and successful synthesis of COFs by condensation reactions of phenyl diboronic acid {C6H4[B(OH)2]2} and hexahydroxytriphenylene [C18H6(OH)6]. Powder X-ray diffraction studies of the highly crystalline products (C3H2BO)6&(C9H12)1 (COF-1) and C9H4BO2 (COF-5) revealed 2-dimensional expanded porous graphitic layers that are either staggered (COF-1, P63/mmc) or eclipsed (COF-5, P6/mmm). Their crystal structures are entirely held by strong bonds between B, C, and O atoms to form rigid porous architectures with pore sizes ranging from 7 to 27 angstroms. COF-1 and COF-5 exhibit high thermal stability (to temperatures up to 500 to 600 C), permanent porosity, and high surface areas (711 and 1590 square meters per gram, respectively).[1]
The synthesis of 3D COFs has been hindered by longstanding practical and conceptual challenges. Unlike 0D and 1D system, the insolubility of 2D and 3D structures precludes the use of stepwise synthesis, making their isolation in crystalline form very difficult. The first challenge, however, was overcome by judiciously choosing building blocks and using reversible condensation reactions to crystallize COFs. Examples of 3D COFs are COF-102, 103, 105, 108, 202, and 300. Most of 3D COF show high surface area, which surpass those of 2D (3472, 4210, 3214, square meters per gram for COF-102, 103, and 202 respectively). COF-105 and 108 calaulated theoretically to perform exceptional hydrogen storage function which is the highest value reported for associative H2 storage of any material.[7]
Synthetic Chemistry of COFs
Boron condensation
The most popular COF synthesis route is a boron condensation reaction which is a molecular dehydration reaction between boronic acids. In case of COF-1, three boronic acid molecules converge to form a planar six-membered B3O3 (boroxine) ring with the elimination of three water molecules.[1]
Triazine based trimerization
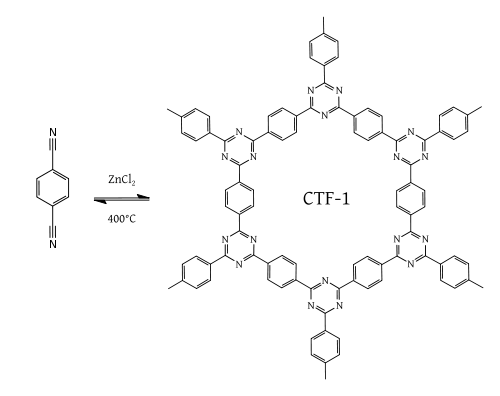
Another class of high performance polymer frameworks with regular porosity and high surface area is based on triazine materials which can be achieved by dynamic trimerization reaction of simple, cheap, and abundant aromatic nitriles in ionothermal conditions (molten zinc chloride at high temperature (400 °C)). CTF-1 is a good example of this chemistry.[8]
Imine condensation
A new class of COFs can be obtained by imine condensation of aniline with benzaldehyde that results in imine bond formation with elimination of water. COF-300 is a good example of this chemistry.[9]
Characterization
Even though COFs are usually harder to characterize properties than MOFs because COFs have no single crystal structure, COFs can be characterized by some following methods. Powder X-ray diffraction (PXDR) is used to determine structure. Morphology is understood by scanning electron microscopy (SEM). Finally, porosity, in most cases surface area, is measured by a N2 sorption isotherm.
See also
References
- ^ a b c d e f Côté, A. P.; Benin, A. I.; Ockwig, N. W.; O'Keeffe, M.; Matzger, A. J.; Yaghi, O. M.; Porous, Crystalline, Covalent Organic Frameworks. Science. 2005, 310, pp 1166-1170. doi:10.1126/science.1120411
- ^ Kitagawa, S.; Kitaura, R.; Noro, S.; Functional Porous Coordination Polymers. Angew. Chem. Int. Ed. 2004, 43, pp 2334-2375. doi:10.1002/anie.200300610
- ^ James, S. L.; Metal-organic frameworks. Chem. Soc. Rev. 2003, 32, pp 276-288. doi:10.1039/B200393G
- ^ a b Yaghi, O. M.; O'Keeffe, M.; Ockwig, N. W.; Chae, H. K.; Eddaoudi, M.; Kim, J.; Reticular synthesis and the design of new materials. Nature. 2003, 423, pp 705-714. doi:10.1038/nature01650
- ^ Han, S.; Hurukawa, H.; Yaghi, O. M.; Goddard, W. A.; Covalent Organic Frameworks as Exceptional Hydrogen Storage Materials. J. Am. Chem. Soc. 2008, 130, pp 11580–11581. doi:10.1021/ja803247y
- ^ Shun, W.; Jia, G.; Jangbae, K.; Hyotcherl, I.; Donglin, J.; A Belt-Shaped, Blue Luminescent, and Semiconducting Covalent Organic Framework. Angew. Chem. Int. Ed. 2008, 47, pp 8826-8830. doi:10.1002/anie.200890235
- ^ El-Kaderi, H. M.; Hunt, J. R.; Mendoza-Cortés, J.; Côté, A.; Taylor, R. E.; O'Keeffe, M.; Yaghi1, O. M.; Designed Synthesis of 3D Covalent Organic Frameworks. Science. 2007, 316, pp 268-272. doi:10.1126/science.1139915
- ^ Kuhn, P.; Antonietti, M.; Thomas, A.; Porous, Covalent Triazine-Based Frameworks Prepared by Ionothermal Synthesis. Angew. Chem. Int. Ed. 2008. 47, pp 3450-3453. PMID 18330878
- ^ Uribe-Romo, F. J.; Hunt, J. R.; Furukawa, H.; Klck, C.; O’Keeffe, M.; Yaghi, O. M.; A Crystalline Imine-Linked 3-D Porous Covalent Organic Framework. J. Am. Chem. Soc. 2009, 131, pp 4570-4571. doi:10.1021/ja8096256
External links
Categories:- Organic compounds
Wikimedia Foundation. 2010.