- Octaazacubane
-
Octaazacubane 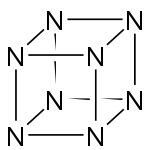
 Other namesOctaazapentacyclo[4.2.0.02,5.03,8.04,7]octane; Cubaazane; Nitrogen octaatomic molecule
Other namesOctaazapentacyclo[4.2.0.02,5.03,8.04,7]octane; Cubaazane; Nitrogen octaatomic moleculeIdentifiers CAS number 78998-15-9 
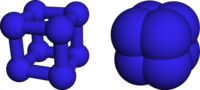
Jmol-3D images Image 1 - N12N3N4N1N5N4N3N52
Properties Molecular formula N8 Molar mass 112.05 g mol−1 Density 2.69 g/cm3 (predicted)[1]  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Octaazacubane is a hypothetical allotrope of nitrogen, whose molecules have eight atoms arranged into a cube. (By comparison, nitrogen usually occurs as the diatomic molecule N2.) It can be regarded as a derivative of cubane, where all eight carbon atoms (and their corresponding hydrogen atoms) have been replaced with a nitrogen atom.[2] It is predicted to be a metastable molecule, in which despite the thermodynamic instability caused by bond strain, and the high energy of the N-N single bonds, the molecule remains kinetically stable for reasons of orbital symmetry.[3]
Explosive and fuel
Octaazacubane is predicted to have an energy density (assuming decomposition into N2) of 22.9 MJ / kg,[4] which is over 5 times the standard value of TNT. It has therefore been proposed (along with other exotic nitrogen allotropes) as an explosive, and as a component of high performance rocket fuel.[5] Its velocity of detonation is predicted to be 15,000 m/s, much (50%) more than any known nonnuclear explosive.[6]
See also
- Tetranitrogen (Nitrogen allotrope with formula N4)
- Hexazine (Nitrogen allotrope with formula N6)
- Octanitrocubane
References
- ^ Agrawal, Jai Prakash (2010). High Energy Materials: Propellants, Explosives and Pyrotechnics. Online: Wiley-VCH. pp. 498. ISBN 9783527628803. http://books.google.com/books?id=rqZROysoS7QC&pg=PA147&lpg=PA147&dq=octaazacubane&source=bl&ots=Ou5l7PbMJm&sig=cHhuquQ5LbBh-KCJfK-k43YRYCA&hl=en&ei=DQiZTtPeCabg0QG50ozFBA&sa=X&oi=book_result&ct=result&resnum=3&ve.
- ^ B. Muir. "Cubane". http://www.ch.ic.ac.uk/local/projects/b_muir/Cubane/Cubanepro/Start.html(See under "further topics" section.)
- ^ Ujwala N. Patil, Nilesh R. Dhumal and Shridhar P. Gejji. "Theoretical studies on the molecular electron densities and electrostatic potentials in azacubanes". Theoretical Chemistry Accounts: Theory, Computation, and Modeling (Theoretica Chimica Acta) 112: p. 27-32. http://www.springerlink.com/content/w3pap8xmmju00j3e/.
- ^ Mikhail N. Glukhovtsev, Haijun Jiao, and Paul von Ragué Schleyer. "Besides N2, What Is the Most Stable Molecule Composed Only of Nitrogen Atoms?". Inorganic Chemistry 35: p. 7124–7133. http://pubs.acs.org/doi/abs/10.1021/ic9606237.
- ^ "Exploding the mysteries of nitrogen.". Chemistry and Industry. http://www.entrepreneur.com/tradejournals/article/60525404.html.
- ^ Agrawal, Jai Prakash (2010). High Energy Materials: Propellants, Explosives and Pyrotechnics. Online: Wiley-VCH. pp. 498. ISBN 9783527628803. http://books.google.com/books?id=rqZROysoS7QC&pg=PA147&lpg=PA147&dq=octaazacubane&source=bl&ots=Ou5l7PbMJm&sig=cHhuquQ5LbBh-KCJfK-k43YRYCA&hl=en&ei=DQiZTtPeCabg0QG50ozFBA&sa=X&oi=book_result&ct=result&resnum=3&ve.
Categories:- Nitrogen
- Explosive chemicals
- Chemistry stubs
Wikimedia Foundation. 2010.
