- Norleucine
-
Norleucine 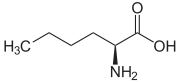 2-aminohexanoic acidOther namesCaprine
2-aminohexanoic acidOther namesCaprine
GlycoleucineIdentifiers CAS number 327-57-1 (2S) PubChem 9475 ChemSpider 401917 UNII 832C8OV84S EC number 210-462-7 DrugBank DB04419 KEGG C01933 MeSH Norleucine ChEBI CHEBI:36405 RTECS number RC6308000 Beilstein Reference 1721748 Gmelin Reference 464584 3DMet B00369 Jmol-3D images Image 1 - CCCCC(N)C(O)=O
- InChI=1S/C6H13NO2/c1-2-3-4-5(7)6(8)9/h5H,2-4,7H2,1H3,(H,8,9)
Key: LRQKBLKVPFOOQJ-UHFFFAOYSA-N
Properties Molecular formula C6H13NO2 Molar mass 131.17 g mol−1 Melting point 301 °C (decomposes) [1]
Solubility in water 16 g/l at 23 °C [2] Acidity (pKa) 2.39 (carboxyl), 9.76 (amino)[3] Hazards S-phrases 36/37 Related compounds Related Aminoacids Norvaline (2-amino-pentanoic)
Aminocaproic acid (6-amino-hexanoic)
Leucine (2-amino-4-methyl-pentanoic)
Isoleucine (2-amino-3-methyl-pentanoic)
Lysine (2,6-diamino-hexanoic)Related compounds Caproic acid (hexanoic)  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Norleucine (abbreviated as Nle) is an isomer of leucine, the α-amino acid 2-amino-hexanoic acid. It is not found in natural proteins. Norleucine is used in experimental studies of protein structure and function.
See also
- Leucines, description of the isomers of leucine
References
- ^ Hermann Römpp, Jürgen Falbe und Manfred Regitz: Römpp Lexikon Chemie, 9. Auflage, Georg Thieme Verlag, Stuttgart 1992.
- ^ Sicherheitsdatenblatt Acros.
- ^ Dawson, R.M.C., et al., Data for Biochemical Research, Oxford, Clarendon Press, 1959.

This article about an organic compound is a stub. You can help Wikipedia by expanding it.
