- Nitroacetic acid
-
Nitroacetic acid 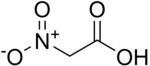 2-Nitroacetic acidOther namesNitro acetate
2-Nitroacetic acidOther namesNitro acetateIdentifiers CAS number 625-75-2 
PubChem 43581 ChEMBL CHEMBL571463 
Jmol-3D images Image 1 - C(C(=O)O)[N+](=O)[O-]
Properties Molecular formula C2H3NO4 Molar mass 105.05 g mol−1 Acidity (pKa) 1.68 [1]  acid (verify) (what is:
acid (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Nitroacetic acid is the chemical compound with the formula (NO2)CH2CO2H. This substituted carboxylic acid is used as a potential precursor to nitromethane, commonly-used as a fuel in drag racing and as an organic reagent in chemical synthesis.
Synthesis
Nitroacetic acid can be synthesized by adding cold chloroacetic acid into a cold, slightly alkaline aqueous solution , followed by mixing with aqueous sodium nitrite solution. It is important during this procedure not to make the solution too alkaline and to keep it cold to prevent the formation of sodium glycolate.
Reactions
Nitroacetic acid can be used in the production of nitromethane by heating a corresponding salt to decarboxylation at 80 °C.[2]
References
- ^ Dippy, J.F.J., Hughes, S.R.C., Rozanski, A., J. Chem. Soc., 1959, 2492.
- ^ Organic Syntheses, Coll. Vol. 1, p.401 (1941); Vol. 3, p.83 (1923)
Categories:- Acetic acids
- Nitroethanes
Wikimedia Foundation. 2010.
