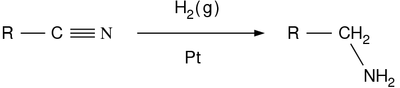
- Nitrile reduction
-
In nitrile reduction a nitrile is reduced to either an amine or an aldehyde with a suitable chemical reagent [1].
Reagents for the conversion to amines are lithium aluminium hydride, Raney nickel / hydrogen / [2] [3] [4] or diborane [5] This organic reaction is one of several nitrogen-hydrogen bond forming reactions.
Nitriles can also be reduced to aldehydes. One method is called the Stephen aldehyde synthesis (Tin(II) chloride, hydrochloric acid and hydrolysis of the iminum salt). Aldehydes also form by reduction with hydrogen with in-situ hydrolysis of the imine. Reagents are Raney nickel [6], lithium aluminium hydride and sodium borohydride.References
- ^ March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (3rd ed.), New York: Wiley, ISBN 0-471-85472-7
- ^ Organic Syntheses, Coll. Vol. 3, p.229 (1955); Vol. 27, p.18 (1947). Link
- ^ Organic Syntheses, Coll. Vol. 3, p.358 (1955); Vol. 27, p.33 (1947). Link
- ^ Organic Syntheses, Coll. Vol. 3, p.720 (1955); Vol. 23, p.71 (1943). Link
- ^ Organic Syntheses, Coll. Vol. 6, p.223 (1988); Vol. 53, p.21 (1973). Link
- ^ Organic Syntheses, Coll. Vol. 6, p.631 (1988); Vol. 51, p.20 (1971). Link

This organic chemistry article is a stub. You can help Wikipedia by expanding it.