- Monocerin
-
Monocerin 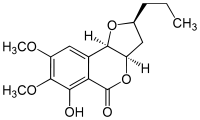 (2S,3aR,9bR)-6-hydroxy-7,8-dimethoxy-2-propyl-2,3,3a,9b-tetrahydro-5H-furo[3,2-c]isochromen-5-one
(2S,3aR,9bR)-6-hydroxy-7,8-dimethoxy-2-propyl-2,3,3a,9b-tetrahydro-5H-furo[3,2-c]isochromen-5-oneIdentifiers PubChem 92267 ChemSpider 83301 ChEMBL CHEMBL488513 Jmol-3D images Image 1 - O=C3O[C@H]1[C@H](O[C@@H](CCC)C1)c2c3c(O)c(OC)c(OC)c2
- InChI=InChI=1S/C16H20O6/c1-4-5-8-6-11-14(21-8)9-7-10(19-2)15(20-3)13(17)12(9)16(18)22-11/h7-8,11,14,17H,4-6H2,1-3H3/t8-,11+,14+/m0/s1
Properties Molecular formula C16H20O6 Molar mass 308.33 g/mol Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references Monocerin is a polyketide metabolite that originates from various fungal species. It has been shown to display antifungal, plant pathogenic, and insecticidal characteristics. Monocerin has been isolated from Dreschlera monoceras,D. ravenelii, Exserohilum turcicum, and Fusarium larvarum.[1]
Contents
Biosynthesis
PKSs of fungi are Type I PKSs. Monocerin has been confirmed by biosynthesis studies to have heptaketide origins. Monocerin PKS is particularly interesting because it produces an intermediate with initially a high degree of reductive modification but ends with a classical β-polyketide moiety. Dihydro isocoumarin is the first PKS free intermediate which would be formed from the reduced heptaketide whose assembly pathway is shown in figure 1.[2] Ketosynthase, ketoreductase, dehydrates, enol reductases and cyclisases are shown as domains of the Monocerin PKS and methyl transferase is considered to be a tailoring enzyme.[3]
 Figure 1. Biosyntheis of Monocerin
Figure 1. Biosyntheis of Monocerin
- Formation of an enolate ion on the carbon three carbons away from sulfur allows aldol addition onto the carbonyl six carbons distant along the chain. This produces the secondary alcohol. Dehydration proceeds to give the alkene. Enolization then occurs to reach the stability of the aromatic ring.[4]
- The modified chain is transferred to the TE domain. This will allow lactonization and release from the enzyme.[4]
- Hydroxylation occurs at ortho-position to two substituents. O-methylation occurs.[1]
- O-methylation
- Cylclic-ether formation
Biological effects
Monocerin produced by Exserohilum turcicum causes Northern Corn Leaf blight disease in maize. The maize will develop brown lesions on its leaves and will have decreased viability in its root cap cells.[5] Monocerin has also been shown to be an effective insecticide against wooly aphids.[6] Monocerin is also an effective herbicide against Johnson grass by inhibiting seedling growth. It has a lesser effect against cucumber.[7]
See also
- Polyketide synthase
- Fungicide use in the United States
- Setosphaeria turcica
References
- ^ a b Axford, L.C., Simpson, T.J., Willis, C.L. (2004). "Synthesis and Incorporation of the First Polyketide Synthase Free Intermediate in Monocerin Biosynthesis". Angenwandte Chemie. 116: 745–748.
- ^ Weerasooriya, M.K.B. and Crosyb, J. (2007). "METHYL TRANSFERASE, A POLYKETIDE BIOSYNTHETIC ENZYME FROM DRESCHLERA MONOCERAS: PURIFICATION AND PROPERTIES". J. Sci. Univ. Kelaniya. 3: 1–16.
- ^ Staunton, J., Weissman, K.J. (2001). "Polyketide biosyntheis: a millenium review". Natural Product Report 18: 380–416.
- ^ a b Dewick, P.M. (2009). Medicinal Natural Products, 3rd. Ed.. Wiley. pp. 101.
- ^ Cuq, F., Brown, S.C., Petitprez, M., and Alibert, G. (1995). "Effects of monocerin on cell cycle progression in maize root meristems synchronized with aphidicolin". Plant Cell Reports 15: 138–142.
- ^ Grove, J.F. and Pople M. (1979). "Metabolic Products of Fusarium larvarum Fuckel. The Fusarentins and the Absolute configuration of Monocerin". Journal of the Chemical Society, Perkin Transactions 1 1: 2048–2051.
- ^ Roberson, D.J. and Strobel, G.M. (1982). "Monocerin, a Phytotoxin from Exserohilum turcicum". Agricultural Biological Chemistry 46: 2681–2683.
Categories:- Plant toxin insecticides
- Natural products
- Polyketide antibiotics
Wikimedia Foundation. 2010.
