- Disulfur difluoride
-
Disulfur difluoride 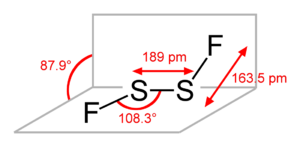 fluorosulfanyl thiohypofluorite
fluorosulfanyl thiohypofluoriteIdentifiers PubChem 123323 ChemSpider 109926 Jmol-3D images Image 1 - FSSF
- InChI=InChI=1S/F2S2/c1-3-4-2
Properties Molecular formula S2F2 Molar mass 102.127 Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references Disulfur difluoride is a halide of sulfur, with the chemical formula S2F2. Silver(II) fluoride can fluorinate sulfur in a strictly dry container, and the reaction produces FS-SF:
Disulfur difluoride will undergo intramolecular rearrangement with the existence of alkali elements' fluorides, obtaining the isomer S=SF2:
S=SF2 can be synthesized with the reaction of potassium fluorosulfinate and sulfur dichloride:
References
Categories:- Inorganic compound stubs
- Sulfur compounds
- Fluorides
Wikimedia Foundation. 2010.



