- Dihydroxymethylidene
-
Dihydroxymethylidene 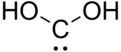
 DihydroxymethylideneSystematic nameDihydroxymethylidene[citation needed] (substitutive)
DihydroxymethylideneSystematic nameDihydroxymethylidene[citation needed] (substitutive)
Dihydroxidocarbon(2•)[citation needed] (additive)Other namesCarbonic(II) acid
Carbonous acid
Dihydroxymethylene
DihydroxycarbeneIdentifiers CAS number 71946-83-3 
PubChem 191992 ChemSpider 11646598 
MeSH Dihydroxycarbene Jmol-3D images Image 1 - O[C]O
Properties Molecular formula CH2O2 Molar mass 46.03 g mol−1 Exact mass 46.005479308 g mol-1 Related compounds Related compounds Lead(II) hydroxide
Orthocarbonic acid
Tin(II) hydroxideExcept where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references Dihydroxymethylidene is the chemical compound with the formula CH2O2. There is no evidence that dihydroxymethylidene exists in solution, but the molecule has been detected in the gas phase.
It is produced in the gas phase by neutralization of the dihydroxymethaniumyl radical cation which is formed by dissociative electron ionization of oxalic acid. Reionization shows that dihydroxymethylidene can survive intact, and thus exists as a stable species with appreciable barriers for dissociation or rearrangement to formic acid. During the production of dihydroxymethylidene, a small fraction of the methylidene decomposes to water and carbon monoxide. Comparison of experimental results with ab initio theory shows that the dissociating molecules are generated in the electronically excited triplet state, while the large amount of surviving carbene molecules is formed in the singlet ground state. Alternatively, dihydroxymethylidene can be produced via high vacuum flash pyrolysis of oxalic acid.
In high concentrations, dihydroxymethylidene has a tendency to, through a series of steps, convert to glyoxylic acid. These steps are as follows:
- 2 dihydroxymethylidene → ethene-1,1,2,2-tetrol → glyoxylic acid
According to the older system which uses prefixes and suffixes to indicate the oxidation number in oxyacids, dihydroxymethylidene can be called carbonous acid. The corresponding mono- and di- deprotonated anions would be called bicarbonite and carbonite. Despite this convention, it is unlikely that this substance reacts as an acid in the conventional sense of an oxyacid.
This article about an organic compound is a stub. You can help Wikipedia by expanding it.
