- Curium(III) oxide
-
Curium(III) oxide 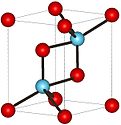 Curium(III) oxideSystematic nameCurium(3+) oxideOther namesCuric oxide
Curium(III) oxideSystematic nameCurium(3+) oxideOther namesCuric oxide
Curium sesquioxideIdentifiers CAS number 12371-27-6 
Jmol-3D images Image 1 - [O--].[O--].[O--].[Cm+3].[Cm+3]
Properties Molecular formula Cm2O3 Molar mass 542.14 g mol−1 Exact mass 541.985 g mol-1 Structure Crystal structure Hexagonal, hP5 Space group P-3m1, No. 164 Related compounds Other cations Gadolinium(III) oxide  oxide (verify) (what is:
oxide (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Curium(III) oxide is a compound composed of curium and oxygen with chemical formula Cm2O3. Curium forms two oxides, curium(III) oxide (Cm2O3) and curium(IV) oxide (CmO2). However, curium(III) oxide is more common. Both curium oxides are solids, insoluble in water but soluble in mineral acids.[1]
See also
References
- ^ Norman M. Edelstein, James D. Navratil, Wallace W. Schulz (1984). Americium and curium chemistry and technology. D. Reidel Pub. Co.. pp. 167-168.
External links
Curium compounds Cm(OH)3 · Cm2O3
Categories:- Inorganic compound stubs
- Curium compounds
- Sesquioxides
Wikimedia Foundation. 2010.
