- Fidaxomicin
-
Fidaxomicin 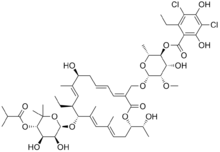
Systematic (IUPAC) name 3-(((6-Deoxy-4-O-(3,5-dichloro-2-ethyl-4,6-dihydroxybenzoyl)-2-O-methyl-β-D-mannopyranosyl)oxy)-methyl)-12(R)-[(6-deoxy-5-C-methyl-4-O-(2-methyl-1-oxopropyl)-β-D-lyxo-hexopyranosyl)oxy]-11(S)-ethyl-8(S)-hydroxy-18(S)-(1(R)-hydroxyethyl)-9,13,15-trimethyloxacyclooctadeca-3,5,9,13,15-pentaene-2-one Clinical data Licence data US FDA:link Pregnancy cat. B(US) Legal status ℞-only (US) Routes Oral Pharmacokinetic data Half-life 11.7 ± 4.80 hours Excretion Feces (92%), urine (0.59%)[1] Identifiers CAS number 873857-62-6 
ATC code None PubChem CID 11528171 UNII Z5N076G8YQ 
KEGG D09394 
ChEMBL CHEMBL1255800 
Synonyms Clostomicin B1, lipiarmicin, lipiarmycin, lipiarmycin A3, OPT 80, PAR 01, PAR 101, tiacumicin B Chemical data Formula C52H74Cl2O18 Mol. mass 1058.04 g/mol  (what is this?) (verify)
(what is this?) (verify)Fidaxomicin (trade name Dificid, and previously OPT-80 and PAR-101) is the first in a new class of narrow spectrum macrocyclic antibiotic drugs.[2] It is a fermentation product obtained from the actinomycete Dactylosporangium aurantiacum subspecies hamdenesis.[1][3] Fidaxomicin is non-systemic, meaning it is minimally absorbed into the bloodstream, it is bactericidal, and it has demonstrated selective eradication of pathogenic Clostridium difficile with minimal disruption to the multiple species of bacteria that make up the normal, healthy intestinal flora. The maintenance of normal physiological conditions in the colon can reduce the probability of Clostridium difficile infection recurrence.[4][5]
It is marketed by Optimer Pharmaceuticals for treatment of Clostridium difficile infection. Fidaxomicin is available in a 200mg tablet that is administered every 12 hours for a recommended duration of 10 days. Total duration of therapy should be determined by the patient's clinical status.
It works by inhibiting the bacterial enzyme RNA polymerase, resulting in the death of Clostridium difficile.[6] It is active against gram positive bacteria especially clostridia. The minimal inhibitory concentration (MIC) range for C. difficile (ATCC 700057) is 0.03–0.25(μg/mL).[1]
Clinical trials
Good results were reported in 2009 from a North American phase III trial comparing it with oral vancomycin for the treatment of Clostridium difficile infection (CDI)[7][8] The study met its primary endpoint of clinical cure, showing that fidaxomicin was non-inferior to oral vancomycin (92.1% vs. 89.8%). In addition, the study met its secondary endpoint of recurrence: 13.3% of the subjects had a recurrence with fidaxomicin vs. 24.0% with oral vancomycin. The study also met its exploratory endpoint of global cure (77.7% for fidaxomicin vs. 67.1% for vancomycin).[9] Clinical cure was defined as patients requiring no further CDI therapy two days after completion of study medication. Global cure was defined as patients who were cured at the end of therapy and did not have a recurrence in the next 4 weeks.[10]
Fidaxomicin was shown to be as good as the current standard-of-care, vancomycin, for treating CDI in a Phase III trial published in February of 2011.[11] The authors also reported significantly fewer recurrences of infection, a frequent problem with C. difficile, and similar drug side effects.
The drug won an FDA advisory panel's unanimous approval on April 10, 2011.[12] and full FDA approval on May 27, 2011[13].
References
- ^ a b c Optimer Pharmaceuticals, Inc. Dificid, Full Prescribing Information, NDA 201,699. 2011.
- ^ Revill, P.; Serradell, N.; Bolos, J. (2006). "Tiacumicin B: macrolide antibiotic treatment of C. difficile-associated diarrhea". Drugs of the Future 31 (6): 494–497.
- ^ Adis R&D Profiles Fidaxomicin: Difimicin; Lipiarmycin; OPT 80; OPT-80; PAR 101; PAR-101. Drugs in R & D (Open Access): 28 May 2010 - Volume 10 - Issue 1 - pp 37-45.
- ^ Louie, Thomas; Judy Emery, Walter Krulicki, Brendan Byrne, and Manuel Mah (2009-01). "OPT-80 Eliminates Clostridium difficile and Is Sparing of Bacteroides Species during Treatment of C. difficile Infection". Antimicrobial Agents and Chemotherapy 535 (1): 261–263. doi:10.1128/AAC.01443-07. PMC 2612159. PMID 18955523. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2612159.
- ^ Johnson, Stuart (2009-06). "Recurrent Clostridium difficile infection: a review of risk factors, treatments, and outcomes". Journal of Infection 58 (6): 403–410. doi:10.1016/j.jinf.2009.03.010. PMID 19394704. http://www.sciencedirect.com/science?_ob=PublicationURL&_tockey=%23TOC%236887%232009%23999419993%231131057%23FLA%23&_cdi=6887&_pubType=J&_auth=y&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=d064db6774b6e2dc79bf19d1bd1dc9d5. Retrieved 2009-11-10.
- ^ Srivastava, A.; Talaue, M.; Liu, S.; Degen, D.; Ebright, R. Y.; Sineva, E.; Chakraborty, A.; Druzhinin, S. Y. et al. (2011). "New target for inhibition of bacterial RNA polymerase: 'switch region'". Current Opinion in Microbiology. doi:10.1016/j.mib.2011.07.030. PMID 21862392.
- ^ Optimer's North American phase 3 Fidaxomicin study results presented at the 49th ICAAC, The Medical News, September 16, 2009
- ^ Optimer Pharmaceuticals Presents Results From Fidaxomicin Phase 3 Study for the Treatment, Reuters, May 17, 2009
- ^ Golan, Y.; K.M. Mullane, M. Miller, K. Weiss, A. Lentnek, P. Sears, Y.K. Shue, S.L. Gorbach, T.J. Louie (2009-09). "Low Recurrence Rate Among Patients with C. difficile Infection Treated with Fidaxomicin". ICAAC 2009. San Francisco, California: Interscience Conference on Antimicrobial Agents and Chemotherapy. http://www.icaac.org/
- ^ Gorbach, S.; K. Weiss, P. Sears, and J. Pullman (2009-09). "Safety of Fidaxomicin Versus Vancomycin in Treatment of Clostridium difficile Infection". ICAAC 2009. San Francisco, California: Interscience Conference on Antimicrobial Agents and Chemotherapy and American Society for Microbiology. http://www.icaac.org/
- ^ Louie, Thomas J.; Miller, Mark A.; Mullane, Kathleen M.; Weiss, Karl; Lentnek, Arnold; Golan, Yoav; Gorbach, Sherwood; Sears, Pamela et al. (2011). "Fidaxomicin versus Vancomycin for Clostridium difficile Infection". New England Journal of Medicine 364 (5): 422–31. doi:10.1056/NEJMoa0910812. PMID 21288078.
- ^ "Optimer Wins FDA Panel’s Backing for Antibiotic Fidaxomicin, Bloomberg,"]. Apr 5, 2011. http://www.bloomberg.com/news/2011-04-05/optimer-wins-fda-advisory-panel-s-backing-for-fidaxomicin-1-.html.
- ^ "Dificid (fidaxomicin) Approved For Clostridium Difficile-Associated Diarrhea". May 2011. http://www.medicalnewstoday.com/articles/226796.php.
Categories:- Macrolide antibiotics
Wikimedia Foundation. 2010.
