- NCS-382
-
NCS-382 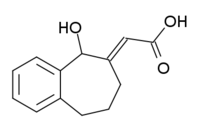 (2E)-(5-hydroxy-5,7,8,9-tetrahydro-6H-benzo[a][7]annulen-6-ylidene ethanoic acid
(2E)-(5-hydroxy-5,7,8,9-tetrahydro-6H-benzo[a][7]annulen-6-ylidene ethanoic acidIdentifiers CAS number 131733-92-1 PubChem 23714994 MeSH NCS-382 Jmol-3D images Image 1 - C1CC2=CC=CC=C2C(C(=CC(=O)O)C1)O
Properties Molecular formula C13H14O3 Molar mass 218.248  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references NCS-382 is a moderately selective antagonist for the GHB receptor.[1][2] It blocks the effects of GHB in animals and has both anti-sedative and anticonvulsant effects.[3][4][5] It has been proposed as a treatment for GHB overdose in humans, as well as for the endogenous metabolic disorder succinic semialdehyde dehydrogenase deficiency, but has never been developed for clinical use.[6]
References
- ^ Castelli MP, Pibiri F, Carboni G, Piras AP. A review of pharmacology of NCS-382, a putative antagonist of gamma-hydroxybutyric acid (GHB) receptor. CNS Drug Reviews. 2004 Fall;10(3):243-60. PMID 15492774
- ^ Ticku MK, Mehta AK (October 2008). "Characterization and pharmacology of the GHB receptor". Annals of the New York Academy of Sciences 1139: 374–85. doi:10.1196/annals.1432.048. PMID 18991884.
- ^ Maitre M, Hechler V, Vayer P, Gobaille S, Cash CD, Schmitt M, Bourguignon JJ. A specific gamma-hydroxybutyrate receptor ligand possesses both antagonistic and anticonvulsant properties. Journal of Pharmacology and Experimental Therapeutics. 1990 Nov;255(2):657-63. PMID 2173754
- ^ Schmidt C, Gobaille S, Hechler V, Schmitt M, Bourguignon JJ, Maitre M. Anti-sedative and anti-cataleptic properties of NCS-382, a gamma-hydroxybutyrate receptor antagonist. European Journal of Pharmacology. 1991 Oct 22;203(3):393-7. PMID 1773824
- ^ Colombo G, Agabio R, Bourguignon J, Fadda F, Lobina C, Maitre M, Reali R, Schmitt M, Gessa GL. Blockade of the discriminative stimulus effects of gamma-hydroxybutyric acid (GHB) by the GHB receptor antagonist NCS-382. Physiology and Behaviour. 1995 Sep;58(3):587-90. PMID 8587968
- ^ Gupta M, Greven R, Jansen EE, Jakobs C, Hogema BM, Froestl W, Snead OC, Bartels H, Grompe M, Gibson KM. Therapeutic intervention in mice deficient for succinate semialdehyde dehydrogenase (gamma-hydroxybutyric aciduria). Journal of Pharmacology and Experimental Therapeutics. 2002 Jul;302(1):180-7. PMID 12065715
GHBergics Receptor
ligands1,4-BD • 4-Methyl-GHB • GABOB • GBL • GHB • GHV • GVL • NCS-356 • NCS-435 • T-HCA • UMB66 • UMB68 • UMB72 • UMB86; Benzamides: Amisulpride • Sulpiride • SultoprideNCS-382
This anticonvulsant-related article is a stub. You can help Wikipedia by expanding it.
