- Pentylamine
-
Pentylamine[1] 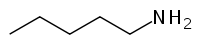 pentanamineOther namesPentanamine, n-Amylamine, 1-Aminopentane
pentanamineOther namesPentanamine, n-Amylamine, 1-AminopentaneIdentifiers CAS number 110-58-7 
PubChem 8060 ChemSpider 7769 
EC number 203-780-2 DrugBank DB02045 ChEMBL CHEMBL1230970 
Jmol-3D images Image 1 - NCCCCC
Properties Molecular formula C5H13N Molar mass 87.16 g/mol Density 0.755 g/cm3 Melting point -50 °C, 223 K, -58 °F
Boiling point 104 °C, 377 K, 219 °F
Solubility in water Miscible Hazards R-phrases R11, R20, R21, R22, R34 S-phrases S16, S26, S36, S37, S39, S45 Main hazards corrosive, highly flammable Flash point 4 °C Related compounds Related compounds butylamine  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Pentylamine is a chemical compound with the formula CH3(CH2)4NH2. It is used as a solvent, as a raw material in the manufacture of a variety of other compounds, including dyes, emulsifiers, and pharmaceutical products,[2] and as a flavoring agent.[3]
References
- ^ Chemblink
- ^ Flick, Ernest W. (1998). Industrial Solvents Handbook (5th ed. ed.). Park Ridge, NJ: William Andrew. pp. 695. ISBN 0-8155-1413-1.
- ^ "JECFA Evaluations-PENTYLAMINE. Summary of Evaluations Performed by the Joint FAO/WHO Expert Committee on Food Additives" (January 31, 2006). Retrieved on 2008-07-25

This article about an amine is a stub. You can help Wikipedia by expanding it.
