- Osmium(IV) chloride
-
Osmium(IV) chloride 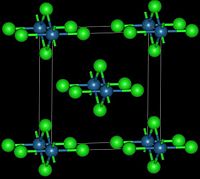 Osmium(IV) chlorideOther namesOsmium chloride, osmium tetrachloride
Osmium(IV) chlorideOther namesOsmium chloride, osmium tetrachlorideIdentifiers CAS number 10026-01-4 Properties Molecular formula OsCl4 Molar mass 332.041 g/mol Appearance red-black orthorhombic crystals Density 4.38 g/cm³ Melting point decomposes at 323°C
Solubility in water reacts with water Solubility soluble in hydrochloric acid Structure Crystal structure Orthorhombic, oS10 Space group Cmmm, No. 65 Related compounds Other anions Osmium(IV) oxide Other cations Iron(III) chloride
Ruthenium(III) chloride
Osmium(III) chloride (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Osmium(IV) chloride or osmium tetrachloride is the inorganic compound composed of osmium and chlorine with the empirical formula OsCl4. It exists in two polymorphs (crystalline forms). The compound is used to prepare other osmium complexes.
Preparation, structure, reactions
It was first reported in 1909 as the product of chlorination of osmium metal.[1] This route affords the high temperature polymorph:[2]
- Os + 2 Cl2 → OsCl4
This reddish-black polymorph is orthorhombic and adopts a structure in which osmium centres are octahedrally coordinated, sharing opposite edges of the OsCl6 octahedra to form a chain.[3] A brown, apparently cubic polymorph forms upon reduction of osmium tetroxide with thionyl chloride:[4]
- OsO4 + 4 SOCl2 → OsCl4 + 2 Cl2 + 4 SO2
Osmium tetraoxide dissolves in hydrochloric acid to give the hexachloroosmate anion:
- OsO4 + 8 HCl → H2OsCl6 + Cl2 + 4 H2O
References
- ^ Otto Ruff and Ferd. Bornemann (1910). "Über das Osmium, seine analytische Bestimmung, seine Oxyde und seine Chloride"". Zeitschrift für anorganische Chemie 65: 429–456. doi:10.1002/zaac.19100650126.
- ^ Cotton, S. A. (1997). Chemistry of Precious Metals. London: Chapman and Hall. ISBN 0751404136.
- ^ Wells A.F. (1984). Structural Inorganic Chemistry (5th ed.). Oxford Science Publications. ISBN 0-19-855370-6.
- ^ Paul Machmer (1967). "On the polymorphism of osmium tetrachloride". Chem. Commun. (12): 610a–610a. doi:10.1039/C1967000610A.
Osmium compounds OsO2 · OsO4 · OsCl4 · Os3(CO)12 · H2Os3(CO)10 · C10H10Os
Categories:- Inorganic compound stubs
- Osmium compounds
- Chlorides
- Metal halides
Wikimedia Foundation. 2010.
