- Ruboxistaurin
-
Ruboxistaurin 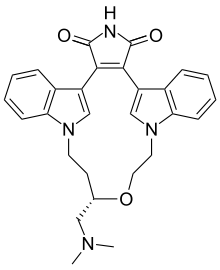
Systematic (IUPAC) name (9S)-9-[(dimethylamino)methyl]-6,7,10,11-tetrahydro-9H,18H-5,21:12,17-di(metheno)dibenzo[e,k]pyrrolo[3,4-h][1,4,13]oxadiazacyclohexadecine-18,20-dione Clinical data Pregnancy cat. ? Legal status ? Identifiers CAS number 169939-94-0 
ATC code None PubChem CID 153999 ChemSpider 135727 
UNII 721809WQCP 
ChEMBL CHEMBL91829 
Chemical data Formula C28H28N4O3 Mol. mass 468.546 g/mol  (what is this?) (verify)
(what is this?) (verify)Ruboxistaurin (proposed brand name Arxxant) is an investigational drug for diabetic peripheral retinopathy. It is currently being investigated by Eli Lilly and Company.
On February, 2006, Lilly submitted a New Drug Application for ruboxistaurin, and on August 18, 2006, Lilly received an "approvable" letter from the United States Food and Drug Administration for ruboxistaurin,[1] with a request for an additional clinical trial, which would take 5 years to complete.[2]
Mechanism of action
Ruboxistaurin is an inhibitor of protein kinase C-beta.[3]
References
- ^ "Drugs.com, Eli Lilly and Company Announces Approvable Letter Issued by FDA for Arxxant". http://www.drugs.com/nda/arxxant_060818.html. Retrieved 2008-02-15.
- ^ "Drugs.com, Lilly Announces FDA Requirement of Additional Clinical Trial Before Ruboxistaurin Could Be Approved for Treatment of Diabetic Retinopathy". http://www.drugs.com/nda/arxxant_060929.html. Retrieved 2008-02-15.
- ^ Clarke M, Dodson PM (December 2007). "PKC inhibition and diabetic microvascular complications". Best Pract Res Clin Endocrinol Metab 21 (4): 573–86. doi:10.1016/j.beem.2007.09.007. PMID 18054736.
External links
This drug article relating to the gastrointestinal system is a stub. You can help Wikipedia by expanding it.
