- Disulfide bond formation protein B
-
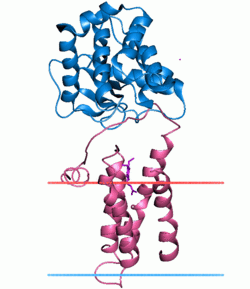
Disulfide bond formation protein B (red), complex with DsbA (blue) Identifiers Symbol DsbB Pfam PF02600 InterPro IPR003752 TCDB 5.A.2 OPM family 194 OPM protein 2hi7 Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary Disulfide bond formation protein B (DsbB) is a protein component of the pathway that leads to disulfide bond formation in periplasmic proteins of Escherichia coli and other bacteria.
The DsbB protein oxidizes the periplasmic protein DsbA which in turn oxidizes cysteines in other periplasmic proteins in order to make disulfide bonds[1]. DsbB acts as a redox potential transducer across the cytoplasmic membrane. It is a membrane protein which spans the membrane four times with both the N- and C-termini of the protein are in the cytoplasm. Each of the periplasmic domains of the protein has two essential cysteines. The two cysteines in the first periplasmic domain are in a Cys-X-Y-Cys configuration that is characteristic of the active site of other proteins involved in disulfide bond formation, including DsbA and protein disulfide isomerase[2].
References
- ^ Lee JO, Beckwith J, Bardwell JC, Jander G, Martin N, Belin D (1993). "A pathway for disulfide bond formation in vivo". Proc. Natl. Acad. Sci. U.S.A. 90 (3): 1038–1042. doi:10.1073/pnas.90.3.1038. PMC 45806. PMID 8430071. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=45806.
- ^ Beckwith J, Jander G, Martin NL (1994). "Two cysteines in each periplasmic domain of the membrane protein DsbB are required for its function in protein disulfide bond formation". EMBO J. 13 (21): 5121–5127. PMC 395459. PMID 7957076. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=395459.
This article includes text from the public domain Pfam and InterPro IPR003752

This membrane protein-related article is a stub. You can help Wikipedia by expanding it.
