- Class II bacteriocin
-
Class II bacteriocin 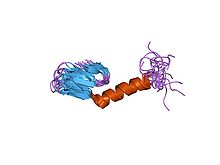
Three-dimensional structure of leucocin A a type IIa bacteriocin.[1] Identifiers Symbol Bacteriocin_II Pfam PF01721 InterPro IPR002633 PROSITE PDOC60030 SCOP 3leu TCDB 1.C.24 OPM family 150 OPM protein 1ohm Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary Class II bacteriocins are a class of small peptides that inhibit the growth of various bacteria.
Many Gram-positive bacteria produce ribosomally synthesized antimicrobial peptides, termed bacteriocins.
Bacteriocins for which disulfide bonds are the only modification to the peptide are Class II bacteriocins.
Contents
Class IIa
One important and well studied class of bacteriocins is the class IIa or pediocin-like bacteriocins produced by lactic acid bacteria. All class IIa bacteriocins are produced by food-associated strains, isolated from a variety of food products of industrial and natural origins, including meat products, dairy products and vegetables. Class IIa bacteriocins are all cationic, display anti-Listeria activity, and kill target cells by permeabilizing the cell membrane[2][3][4].
Class IIa bacteriocins contain between 37 and 48 residues[5]. Based on their primary structures, the peptide chains of class IIa bacteriocins may be divided roughly into two regions: a hydrophilic, cationic and highly conserved N-terminal region, and a less conserved hydrophobic/amphiphilic C-terminal region. The N-terminal region contains the conserved Y-G-N-G-V/L 'pediocin box' motif and two conserved cysteine residues joined by a disulfide bridge. It forms a three-stranded antiparallel beta-sheet supported by the conserved disulfide bridge. This cationic N-terminal beta-sheet domain mediates binding of the class IIa bacteriocin to the target cell membrane. The C-terminal region forms a hairpin-like domain that penetrates into the hydrophobic part of the target cell membrane, thereby mediating leakage through the membrane. The two domains are joined by a hinge, which enables movement of the domains relative to each other[3][4].
Some proteins known to belong to the class IIa bacteriocin family are listed below:
- Pediococcus acidilactici pediocin PA-1.
- Leuconostoc mesenteroides mesentericin Y105.
- Carnobacterium piscicola carnobacteriocin B2.
- Lactobacillus sakei sakacin P.
- Enterococcus faecium enterocin A.
- Enterococcus faecium enterocin P.
- Leuconostoc gelidum leucocin A.
- Lactobacillus curvatus curvacin A.
- Listeria innocua listeriocin 743A.
Class IIb
The class IIb bacteriocins (two-peptide bacteriocins) require two different peptides for activity.
Class IIc
Other class II bacteriocins can be grouped together as Class IIc (circular bacteriocins). These have a wide range of effects on membrane permeability, cell wall formation and pheromone actions of target cells.
References
- ^ Fregeau Gallagher NL, Sailer M, Niemczura WP, Nakashima TT, Stiles ME, Vederas JC (December 1997). "Three-dimensional structure of leucocin A in trifluoroethanol and dodecylphosphocholine micelles: spatial location of residues critical for biological activity in type IIa bacteriocins from lactic acid bacteria". Biochemistry 36 (49): 15062–72. doi:10.1021/bi971263h. PMID 9398233.
- ^ Ennahar S, Sonomoto K, Ishizaki A (1999). "Class IIa bacteriocins from lactic acid bacteria: antibacterial activity and food preservation". J. Biosci. Bioeng. 87 (6): –. doi:10.1016/S1389-1723(99)80142-X. PMID 16232543.
- ^ a b Fimland G, Nissen-Meyer J, Johnsen L (2005). "The C-terminal domain of pediocin-like antimicrobial peptides (class IIa bacteriocins) is involved in specific recognition of the C-terminal part of cognate immunity proteins and in determining the antimicrobial spectrum". J. Biol. Chem. 280 (10): –. doi:10.1074/jbc.M412712200. PMID 15611086.
- ^ a b Dalhus B, Fimland G, Nissen-Meyer J, Johnsen L (2005). "Pediocin-like antimicrobial peptides (class IIa bacteriocins) and their immunity proteins: biosynthesis, structure, and mode of action". J. Pept. Sci. 11 (11): –. doi:10.1002/psc.699. PMID 16059970.
- ^ Simon L, Fremaux C, Cenatiempo Y, Berjeaud JM (2002). "Sakacin g, a new type of antilisterial bacteriocin". Appl. Environ. Microbiol. 68 (12): 6416–20. doi:10.1128/AEM.68.12.6416-6420.2002. PMC 134399. PMID 12450870. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=134399.
Further reading
- [1]. Sequence and structural relationships of leucocins A-, B- and C-TA33a from Leuconostoc mesenteroides TA33a. Papathanasopoulos MA, Dykes GA, Revol-Junelles AM, Delfour A, von Holy A, Hastings JW; Microbiology 1998;144:1343-1348. PubMed
- [2]. Three-dimensional structure of leucocin A in trifluoroethanol and dodecylphosphocholine micelles: spatial location of residues critical for biological activity in type IIa bacteriocins from lactic acid bacteria. Fregeau Gallagher NL, Sailer M, Niemczura WP, Nakashima TT, Stiles ME, Vederas JC; Biochemistry 1997;36:15062-15072. PubMed

This membrane protein-related article is a stub. You can help Wikipedia by expanding it.
