- 4-Chloroindole-3-acetic acid
-
4-Chloroindole-3-acetic acid 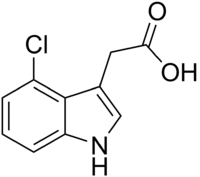 2-(4-chloro-1H-indol-3-yl)acetic acid
2-(4-chloro-1H-indol-3-yl)acetic acidIdentifiers CAS number 2519-61-1 PubChem 100413 ChemSpider 90727 
ChEBI CHEBI:20339 
ChEMBL CHEMBL309993 
Jmol-3D images Image 1
Image 2- OC(CC1=CNC2=CC=CC(Cl)=C21)=O
Clc1cccc2c1c(cn2)CC(=O)O
Properties Molecular formula C10H8ClNO2 Molar mass 209.63 g mol−1  acid (verify) (what is:
acid (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references 4-Chloroindole-3-acetic acid (4-Cl-IAA) is a natural plant hormone.[1] It is a member of the class of compounds known as auxins and a chlorinated derivative of the more common auxin indole-3-acetic acid (IAA). 4-Cl-IAA is found in the seeds of a variety of plants, particularly legumes such as peas and broad beans.[2][3][4][5] It is hypothosized that 4-Cl-IAA may be a "death hormone" that maturing seeds use to trigger death of the parent plant by mobilizing nutrients to be stored in the seed.[6]
References
- ^ Reinecke, Dennis M. 4-Chloroindole-3-acetic acid and plant growth. Plant Growth Regulation (1999), 27(1), 3-13.
- ^ Pless, Tanja; Boettger, Michael; Hedden, Peter; Graebe, Jan (1984). "Occurrence of 4-Cl-indoleacetic acid in broad beans and correlation of its levels with seed development". Plant Physiology 74 (2): 320–3. doi:10.1104/pp.74.2.320. PMC 1066676. PMID 16663416. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1066676.
- ^ Ernstsen, Arild; Sandberg, Goeran (1986). "Identification of 4-chloroindole-3-acetic acid and indole-3-aldehyde in seeds of Pinus sylvestris". Physiologia Plantarum 68 (3): 511–18. doi:10.1111/j.1399-3054.1986.tb03390.x.
- ^ Katayama, Masato; Thiruvikraman, Singanallore V.; Marumo, Shingo (1987). "Identification of 4-chloroindole-3-acetic acid and its methyl ester in immature seeds of Vicia amurensis (the tribe Vicieae), and their absence from three species of Phaseoleae". Plant and Cell Physiology 28 (2): 383–386.
- ^ Magnus, Volker; Ozga, Jocelyn A.; Reinecke, Dennis M.; Pierson, Gerald L.; Larue, Thomas A.; Cohen, Jerry D.; Brenner, Mark L. 4-chloroindole-3-acetic and indole-3-acetic acids in Pisum sativum. Phytochemistry (1997), 46(4), 675-681.
- ^ Engvild, Kjeld C. (1996). "Herbicidal activity of 4-chloroindoleacetic acid and other auxins on pea, barley and mustard". Physiologia Plantarum 96 (2): 333–337. doi:10.1111/j.1399-3054.1996.tb00222.x.
This article about a heterocyclic compound is a stub. You can help Wikipedia by expanding it. - OC(CC1=CNC2=CC=CC(Cl)=C21)=O
