- Diethylpyrocarbonate
-
Diethylpyrocarbonate 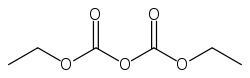
 diethyl dicarbonate
diethyl dicarbonateIdentifiers CAS number 1609-47-8 
PubChem 3051 ChemSpider 2943 
KEGG C11592 
MeSH Diethylpyrocarbonate ChEBI CHEBI:59051 
ChEMBL CHEMBL55517 
Jmol-3D images Image 1 - O=C(OCC)OC(=O)OCC
Properties Molecular formula C6H10O5 Molar mass 162.141 g/mol  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Diethylpyrocarbonate (DEPC), also called diethyl dicarbonate (IUPAC name), diethyl oxydiformate, ethoxyformic anhydride, or pyrocarbonic acid diethyl ester, is used in the laboratory to inactivate the RNase enzymes from water and other laboratory utensils. It inactivates the RNases by the covalent modifications of the histidine residues. DEPC is unstable in water and susceptible to hydrolysis to carbon dioxide and ethanol, especially in the presence of a nucleophile. For this reason DEPC cannot be used with Tris buffer or HEPES. In contrast it can be used with PBS or MOPS. A handy rule is that enzymes or chemicals which have active -O:, -N: or -S: cannot be treated with DEPC to become RNase-free as DEPC reacts with these species. Furthermore DEPC degradation products can inhibit in vitro transcription.
Water is usually treated with 0.1% v/v diethylpyrocarbonate for at least 1hour at 37°C and then autoclaved (at least 15 min) to inactivate traces of DEPC. Inactivation of DEPC in this manner yields CO2, H2O and EtOH. Higher concentrations of DEPC are competent of deactivating larger amounts of RNase but remaining traces or byproducts may inhibit further biochemical reactions such as in vitro translation. Further on, chemical modification of RNA such as carboxymethylation is possible when traces of DEPC or its byproducts are present, resulting to reduced usage of RNA even after buffer exchange (after precipitation).
DEPC treated (and therefore RNase-free) water is used in handling of RNA in the laboratory, to reduce the risk of RNA being degraded by RNases.
DEPC derivatization of histidines is also used to study the importance of histidyl residues in enzymes. Modification of histidine by DEPC results in carbethoxylate derivates at the N-omega-2 nitrogen of the imidazole ring. DEPC modification of histidines can be reversed by treatment with 0.5M hydroxylamine at neutral pH.
External links
Categories:- Biochemistry
- Biochemistry stubs
Wikimedia Foundation. 2010.

