- Monastrol
-
Monastrol 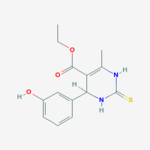
 ethyl 4-(3-hydroxyphenyl)-6-methyl-2-sulfanylidene-3,4-dihydro-1H-pyrimidine-5-carboxylateOther namesMonastrol
ethyl 4-(3-hydroxyphenyl)-6-methyl-2-sulfanylidene-3,4-dihydro-1H-pyrimidine-5-carboxylateOther namesMonastrolIdentifiers PubChem 2987927 ChEMBL CHEMBL236789 
Jmol-3D images Image 1 - CCOC(=O)C1=C(NC(=S)NC1C2=CC(=CC=C2)O)C
- InChI=1S/C14H16N2O3S/c1-3-19-13(18)11-8(2)15-14(20)16-12(11)9-5-4-6-10(17)7-9/h4-7,12,17H,3H2,1-2H3,(H2,15,16,20)
Properties Molecular formula C14H16N2O3S Molar mass 292.35344  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Monastrol is a cell-permeable small molecule inhibitor discovered by Thomas U. Mayer in the lab of Tim Mitchison. Monastrol was shown to inhibit the kinesin Eg5, a motor protein important for spindle bipolarity.[1]
Mechanism of action
Monastrol binds to a long loop that is specific to the Eg5 kinesin family, and allosterically inhibits ATPase activity of the kinesin [2]
References
- ^ Thomas U. Mayer, Tarun M. Kapoor, Stephen J. Haggarty, Randall W. King, Stuart L. Schreiber, Timothy J. Mitchison (1999). "Small Molecule Inhibitor of Mitotic Spindle Bipolarity Identified in a Phenotype-Based Screen". Science 286 (5441): 971–974. doi:10.1126/science.286.5441.971. PMID 10542155. http://www.biochem.wisc.edu/courses/biochem704/Reading/Mayer1999.pdf.
- ^ Maliga Z, Kapoor TM, Mitchison TJ (September 2002). "Evidence that monastrol is an allosteric inhibitor of the mitotic kinesin Eg5". Chem. Biol. 9 (9): 989–96. doi:10.1016/S1074-5521(02)00212-0. PMID 12323373.

This pharmacology-related article is a stub. You can help Wikipedia by expanding it.

