- Convolutindole A
-
Convolutindole A 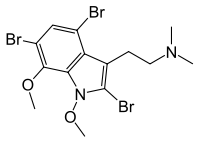 2,4,6-Tribromo-1,7-dimethoxy-N,N-dimethyltryptamine
2,4,6-Tribromo-1,7-dimethoxy-N,N-dimethyltryptamineIdentifiers CAS number 443356-86-3 
Properties Molecular formula C14H17Br3N2O2 Melting point 61.5-62.5 °C
 A (verify) (what is:
A (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Convolutindole A (2,4,6-tribromo-1,7-dimethoxy-N,N-dimethyltryptamine) is a brominated indole alkaloid that was first identified in 2001 in Amathia convoluta, a marine bryozoan. Bryozoans are aquatic invertebrates that grow in colonies and superficially resemble corals.
Chemistry
Convolutamine A is the 2,4,6-tribromo-1,7-dimethoxy analog of DMT, a hallucinogen that occurs naturally in many plants and animals. Other brominated derivatives of DMT include 5-bromo-DMT and 5,6-dibromo-DMT, both of which also occur in marine invertebrates.
The researchers who discovered the chemical drew specific attention to the methoxy group at the indole 1-position (attached to the nitrogen atom in the pentagonal ring) as being an unknown occurrence in the marine world until recently. 1-methoxyindoles also occur in the Brassicaceae, the plant family that cabbage and mustard belong to.
Biological activity
The chemical was tested for its ability to kill nematodes, a type of parasitic worm. It was found to be more effective in this regard than levamisole, a commercial drug used to kill parasitic worms (and in the treatment of colon cancer).
References
- Narkowicz, C. K.; Blackman, A. J., (June 2001). Abstracts of Papers; 10th International Symposium on Marine Natural Products: Nago, Okinawa, Abstract OR1.
- Narkowicz, C. K.; Blackman, A. J.; Lacey, E.; Gill, J. H.; Heiland, K. (2002). "Convolutindole a and Convolutamine H, New Nematocidal Brominated Alkaloids from the Marine BryozoanAmathia convoluta". Journal of Natural Products 65 (6): 938–941. doi:10.1021/np010574x. PMID 12088445.
Categories:- Natural tryptamine alkaloids
- Halogen-containing alkaloids
- Organobromides
- Phenol ethers
Wikimedia Foundation. 2010.
