- Collins reagent
-
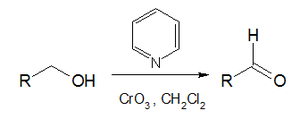
Collins reagent is the complex of chromium(VI) oxide with pyridine in dichloromethane.[1][2] It is used to selectively oxidize primary alcohols to the aldehyde, and will tolerate many other functional groups within the molecule.
It can be used as an alternative to the Jones reagent and pyridinium chlorochromate (PCC) when oxidising secondary alcohols to ketones. Moreover, the Collins reagent is especially useful for oxidations of acid sensitive compounds.
This complex is both difficult and dangerous to prepare, as it is very hygroscopic and can inflame during preparation. It is typically used in a sixfold excess in order to complete the reaction. Nowadays, PCC or PDC oxidation have largely supplanted Collins oxidation for these very reasons.
See also
- Jones oxidation
- Pyridinium chlorochromate
- Pyridinium dichromate
- Sarett oxidation
References
- ^ J. C. Collins, W. W. Hess and F. J. Frank (1968). "Dipyridine-chromium(VI) oxide oxidation of alcohols in dichloromethane". Tetrahedron Lett. 9 (30): 3363–3366. doi:10.1016/S0040-4039(00)89494-0.
- ^ J. C. Collins, W.W. Hess (1988), "Aldehydes from Primary Alcohols by Oxidation with Chromium Trioxide: Heptanal", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv6p0644; Coll. Vol. 6: 644
Categories:- Chemistry stubs
- Oxidizing agents
- Chromium compounds
Wikimedia Foundation. 2010.