- Mesomeric effect
-
See also: Resonance (chemistry)
The mesomeric effect or resonance effect in chemistry is a property of substituents or functional groups in a chemical compound. The effect is used in a qualitative way and describes the electron withdrawing or releasing properties of substituents based on relevant resonance structures and is symbolized by the letter M. The mesomeric effect is negative (-M) when the substituent is an electron-withdrawing group and the effect is positive (+M) when based on resonance and the substituent is an electron releasing group.
- Examples of -M substituents: acetyl (IUPAC ethanoyl) - nitrile - nitro
- Examples of +M substituents: alcohol - amine-benzene
The net electron flow from or to the substituent is determined also by the inductive effect. The mesomeric effect as a result of p-orbital overlap (resonance) has absolutely no effect on this inductive effect, as the inductive effect is purely to do with the electronegativity of the atoms and their topology in the molecule (which atoms are connected to which).
The concepts of mesomeric effect, mesomerism and mesomer were introduced by Ingold in 1938 as an alternative to the Pauling's synonymous concept of resonance.[1] "Mesomerism" in this context is often encountered in German and French literature but in English literature the term "resonance" dominates.
Contents
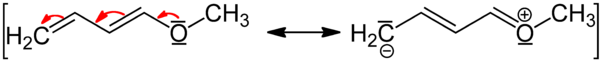
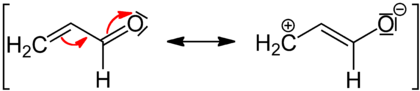
Mesomerism in conjugated systems
Mesomeric effect can be transmitted along any number of carbon atoms in a conjugated system. This accounts for the resonance stabilization of the molecule due to delocalization of charge.
See also
- Important publications in organic chemistry
References
External links
- IUPAC Gold Book definition
Categories:- Chemical bonding
Wikimedia Foundation. 2010.