- Lycorine
-
Lycorine 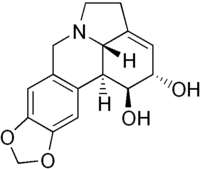 1,2,4,5,12b,12c-Hexahydro-7H-[1,3]dioxolo[4,5-j]pyrrolo[3,2,1-de]phenanthridine-1,2-diolOther namesGalanthidine, Amarylline, Narcissine, Licorine, Belamarine
1,2,4,5,12b,12c-Hexahydro-7H-[1,3]dioxolo[4,5-j]pyrrolo[3,2,1-de]phenanthridine-1,2-diolOther namesGalanthidine, Amarylline, Narcissine, Licorine, BelamarineIdentifiers CAS number 476-28-8 PubChem 72378 ChemSpider 65312 
ChEBI CHEBI:6601 
Jmol-3D images Image 1 - O1c2c(OC1)cc3c(c2)[C@H]4[C@@H]/5N(C3)CCC\5=C/[C@H](O)[C@H]4O
- InChI=1S/C16H17NO4/c18-11-3-8-1-2-17-6-9-4-12-13(21-7-20-12)5-10(9)14(15(8)17)16(11)19/h3-5,11,14-16,18-19H,1-2,6-7H2/t11-,14-,15+,16+/m0/s1

Key: XGVJWXAYKUHDOO-DANNLKNASA-N
InChI=1/C16H17NO4/c18-11-3-8-1-2-17-6-9-4-12-13(21-7-20-12)5-10(9)14(15(8)17)16(11)19/h3-5,11,14-16,18-19H,1-2,6-7H2/t11-,14-,15+,16+/m0/s1
Key: XGVJWXAYKUHDOO-DANNLKNABD
Properties Molecular formula C16H17NO4 Molar mass 287.31 g mol−1  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Lycorine is a toxic crystalline alkaloid found in several plant species, such as the bush lily (Clivia miniata), Lycoris, and Narcissus. It may be highly poisonous, or even lethal, when ingested in certain quantities. Symptoms of lycorine toxicity are vomiting, diarrhea, and convulsions.[1] Regardless, it is sometimes used medicinally, a reason why some groups may harvest the very popular Clivia miniata.
It inhibits protein synthesis,[2] and may inhibit ascorbic acid biosynthesis, although studies on the latter are controversial and inconclusive. Presently, it serves some interest in the study of certain yeasts, the principal organism on which lycorine is tested.[3]
Lycorine is also found in daffodil bulbs which are often confused with onions, thereby leading to incidents of accidental poisoning.[4]
References
- ^ Lycorine, definition at mercksource.com
- ^ Vrijsen R, Vanden Berghe DA, Vlietinck AJ, Boeyé A (1986). "Lycorine: a eukaryotic termination inhibitor?". J. Biol. Chem. 261 (2): 505–7. PMID 3001065.
- ^ Garuccio I, Arrigoni O (1989). "[Various sensitivities of yeasts to lycorine]" (in Italian). Boll. Soc. Ital. Biol. Sper. 65 (6): 501–8. PMID 2611011.
- ^ Pupils ill after bulb put in soup, BBC News, 3 May 2009
External links
- Hill, R. K.; Joule, J. A.; Loeffler, L. J. (1962). "Stereoselective Syntheses of d,l-α- and β-Lycoranes". Journal of the American Chemical Society 84 (24): 4951–4956. doi:10.1021/ja00883a064.
- Wolfgang Oppolzer, Alan C. Spivey, and Christian G. Bochet (1994). "Suprafaciality of Thermal N-4-Alkenylhydroxylamine Cyclizations: Syntheses of (±)-α-Lycorane and (+)-Tianthine". J. Am. Chem. Soc. 116: 3139–3140. doi:10.1021/ja00086a060. http://www.ch.ic.ac.uk/spivey/publicationpdfs/jacs19941163139.pdf.
Categories:- Alkaloids
- Alcohols
Wikimedia Foundation. 2010.
