- Scoulerine
-
Scoulerine 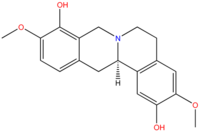 (13aS)-3,10-dimethoxy-5,8,13,13a-tetrahydro-6H-isoquino
(13aS)-3,10-dimethoxy-5,8,13,13a-tetrahydro-6H-isoquino
[3,2-a]isoquinoline-2,9-diolOther namesDiscretamine, Aequaline, Scoulerin.Identifiers CAS number 605-34-5 
PubChem 439654 ChemSpider 388725 
ChEBI CHEBI:17129 
ChEMBL CHEMBL1235966 
Jmol-3D images Image 1 - Oc1c4c(ccc1OC)C[C@H]3c2c(cc(OC)c(O)c2)CCN3C4
- InChI=1S/C19H21NO4/c1-23-17-4-3-11-7-15-13-9-16(21)18(24-2)8-12(13)5-6-20(15)10-14(11)19(17)22/h3-4,8-9,15,21-22H,5-7,10H2,1-2H3/t15-/m0/s1

Key: KNWVMRVOBAFFMH-HNNXBMFYSA-N
InChI=1/C19H21NO4/c1-23-17-4-3-11-7-15-13-9-16(21)18(24-2)8-12(13)5-6-20(15)10-14(11)19(17)22/h3-4,8-9,15,21-22H,5-7,10H2,1-2H3/t15-/m0/s1
Key: KNWVMRVOBAFFMH-HNNXBMFYBO
Properties Molecular formula C19H21NO4 Molar mass 327.37 g/mol  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Scoulerine, also known as discretamine and aequaline, is an alkaloid found in the Opium poppy[1], Croton flavens[2], and certain plants in the Erythrina genus[3]. Studies show that scoulerine is an antagonist at the α2-adrenoceptor, α1D-adrenoceptor and 5-HT receptor[4][5]. It has also been found to be a GABAA receptor agonist[6][2].
References
- ^ Frick S, Chitty JA, Kramell R, Schmidt J, Allen RS, Larkin PJ, Kutchan TM. (2004). "Transformation of opium poppy (Papaver somniferum L.) with antisense berberine bridge enzyme gene (anti-bbe) via somatic embryogenesis results in an altered ratio of alkaloids in latex but not in roots.". Transgenic Res. 13 (6): 607–13. doi:10.1007/s11248-004-2892-6. PMID 15672841.
- ^ a b Eisenreich WJ, Hofner G, Bracher F. (2003). "Alkaloids from Croton flavens L. and their affinities to GABA-receptors.". Nat Prod Res. 17 (6): 437–40. doi:10.1080/1478641031000111516. PMID 14577695.
- ^ Ito K. (1999). "Studies on the alkaloids of Erythrina plants". Yakugaku Zasshi 119 (5): 340–56. PMID 10375996.
- ^ Ko FN, Yu SM, Su MJ, Wu YC, Teng CM. (1993). "Pharmacological activity of (-)-discretamine, a novel vascular alpha-adrenoceptor and 5-hydroxytryptamine receptor antagonist, isolated from Fissistigma glaucescens.". Br J Pharmacol. 110 (2): 882–8. PMC 2175899. PMID 7902181. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2175899.
- ^ Ko FN, Guh JH, Yu SM, Hou YS, Wu YC, Teng CM. (1994). "(-)-Discretamine, a selective alpha 1D-adrenoceptor antagonist, isolated from Fissistigma glaucescens.". Br J Pharmacol. 112 (4): 1174–80. PMC 1910235. PMID 7952879. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1910235.
- ^ Halbsguth C, Meissner O, Haberlein H. (2003). "Positive cooperation of protoberberine type 2 alkaloids from Corydalis cava on the GABA(A) binding site.". Planta Med. 69 (4): 305–9. doi:10.1055/s-2003-38869. PMID 12709895.
External links
This article about a heterocyclic compound is a stub. You can help Wikipedia by expanding it.
