- Ozone-oxygen cycle
-
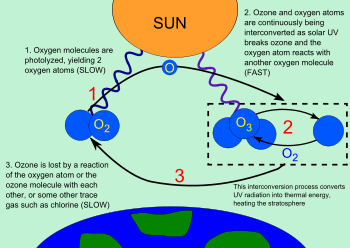
The ozone-oxygen cycle is the process by which ozone is continually regenerated in Earth's stratosphere, all the while converting ultraviolet radiation into heat. In 1930 Sydney Chapman resolved the chemistry involved. The process is commonly called the Chapman cycle by atmospheric scientists.
Most of the ozone production occurs in the tropical upper stratosphere and mesosphere. The total mass of ozone produced per day over the globe is about 400 million metric tons. The global mass of ozone is relatively constant at about 3 billion metric tons, meaning the Sun produces about 12% of the ozone layer each day.[1]
Contents
Chemistry
The ozone molecules formed by the below reaction absorb ultraviolet radiation having wavelengths between 240 and 310 nm. The triatomic ozone molecule becomes diatomic molecular oxygen plus a free oxygen atom:
O3 + (240 nm < radiation < 310 nm) → O2 + O
The atomic oxygen produced immediately reacts with other oxygen molecules to reform ozone:
O2 + O + M → O3 + M
where "M" denotes the third body that carries off the excess energy of the reaction. In this way, the chemical energy released when O and O2 combine is converted into kinetic energy of molecular motion. The overall effect is to convert penetrating UV light into heat, without any net loss of ozone. This cycle keeps the ozone layer in a stable balance while protecting the lower atmosphere from UV radiation, which is harmful to most living beings. It is also one of two major sources of heat in the stratosphere (the other being the kinetic energy released when O2 is photolyzed into O atoms).
Removal
If an oxygen atom and an ozone molecule meet, they recombine to form two oxygen molecules:
O3 + O· → 2 O2
And if two oxygen atoms meet, they react to form one oxygen molecule:
2 O· → O2
The overall amount of ozone in the stratosphere is determined by a balance between production by solar radiation, and removal. The removal rate is slow, since the concentration of O atoms is very low.
Certain free radicals, the most important being hydroxyl (OH), nitric oxide (NO), and atoms of chlorine (Cl) and bromine (Br), catalyze the recombination reaction, leading to an ozone layer that is thinner than it would be if the catalysts were not present.
Most of the OH and NO are naturally present in the stratosphere, but human activity, especially emissions of chlorofluorocarbons (CFCs) and halons, has greatly increased the Cl and Br concentrations, leading to ozone depletion. Each Cl or Br atom can catalyze tens of thousands of decomposition reactions before it is removed from the stratosphere.
External links
References
Categories:
Wikimedia Foundation. 2010.