- Disulfur decafluoride
-
Disulfur decafluoride 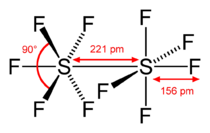

 Disulfur decafluorideSystematic nameDecafluoro-1λ6,2λ6-disulfane
Disulfur decafluorideSystematic nameDecafluoro-1λ6,2λ6-disulfaneIdentifiers CAS number 5714-22-7 PubChem 62586 
ChemSpider 56348 
EC number 227-204-4 MeSH Disulfur+decafluoride Jmol-3D images Image 1 - FS(F)(F)(F)(F)S(F)(F)(F)(F)F
- InChI=1S/F10S2/c1-11(2,3,4,5)12(6,7,8,9)10
Key: BPFZRKQDXVZTFD-UHFFFAOYSA-N
Properties Molecular formula S2F10 Appearance colorless liquid Melting point -53 °C
Boiling point 30.1 °C
Hazards NFPA 704 Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references Disulfur decafluoride (S2F10) is a gas discovered in 1934 by Denbigh and Whytlaw-Gray.[1] Each S of the S2F10 molecule is octahedral, and surrounded by 5 fluorines.[2] S2F10 is highly toxic, with toxicity similar to phosgene. It was considered a potential chemical warfare pulmonary agent in World War II because it does not produce lacrimation or skin irritation, thus providing little warning of exposure. It is a possible by-product of electrically decomposed SF6 gas -- an essentially inert insulator used in high voltage systems such as transmission lines, substations and switchgear. S2F10 is also made during the production of SF6, but is distilled out.
Contents
Properties
This compound contains sulfur in the +5 oxidation state.
At temperatures above 150°C, S2F10 decomposes slowly to SF6 and SF4.
S2F10 reacts with N2F4 to give SF5NF2. It reacts with SO2 to form SF5OSO2F in the presence of ultraviolet radiation.
In the presence of excess chlorine gas, S2F10 reacts to form sulfur chloride pentafluoride (SF5Cl):
- S2F10 + Cl2 → 2 SF5Cl
The analogous reaction with bromine is reversible and yields SF5Br.[3] The reversibility of this reaction can be used to synthesize S2F10 from SF5Br.[4]
Ammonia is oxidised by S2F10 into NSF3.[5]
Toxicity
S2F10 is a colorless, odorless liquid about 4 times as poisonous as phosgene; a single breath can kill within a day. Its toxicity is thought to be caused by its disproportionation in the lungs into SF6, which is inert, and SF4, which reacts with moisture to form sulfurous acid and hydrofluoric acid.[6]
External links
References
- ^ Kenneth G. Denbigh and Robert Whytlaw-Gray (1934). "The preparation and properties of disulphur decafluoride". J. Chem. Soc.: 1346–1352. doi:10.1039/JR9340001346.
- ^ Harvey, R. B.; Bauer, S. H. (June 1953). "An Electron Diffraction Study of Disulfur Decafluoride". Journal of the American Chemical Society 75 (12): 2840–2846. doi:10.1021/ja01108a015.
- ^ Cohen, B.; MacDiarmid, A. G. (December 1965). "Chemical Properties of Disulfur Decafluoride". Inorganic Chemistry 4 (12): 1782–1785. doi:10.1021/ic50034a025.
- ^ Winter, R.; Nixon, P.; Gard, G. (January 1998). "A new preparation of disulfur decafluoride". Journal of Fluorine Chemistry 87 (1): 85–86. doi:10.1016/S0022-1139(97)00096-1.
- ^ Steve Mitchell (1996). Steve Mitchell. ed. Biological interactions of sulfur compounds. CRC Press. p. 14. ISBN 0748402454.
- ^ Harold Johnston (2003). A bridge not attacked: chemical warfare civilian research during World War II. World Scientific. pp. 33–36. ISBN 9812381538.
- Loucas G. Christophorou; Isidor Sauers (1991). Christophorou, Loucas G., Isidor Sauers. ed. Gaseous Dielectrics VI. Plenum Press. ISBN 0-306-43894-1.
Categories:- Pulmonary agents
- Sulfur fluorides
- Inorganic compound stubs
Wikimedia Foundation. 2010.

