- Oxygenation (environmental)
-
See also: oxygen saturation and water aeration
Environmental oxygenation can be important to the sustainability of a particular ecosystem. Insufficient oxygen (environmental hypoxia) may occur in bodies of water such as ponds and rivers, tending to suppress the presence of aerobic organisms such as fish. Deoxygenation increases the relative population of anaerobic organisms such as plants and some bacteria, resulting in fish kills and other adverse events. The net effect is to alter the balance of nature by increasing the concentration of anaerobic over aerobic species.
Oxygenation by water aeration can be part of the environmental remediation of a usually stagnant body of water. For example, Bubbly Creek in Chicago, Illinois, was hypoxic (deficient in oxygen) due to its use as an open sewer by Chicago's meat packing industry but has been oxygenated by introducing compressed air into its waters, increasing the fish population.[citation needed] A similar technique has previously been used in the Thames.[citation needed]
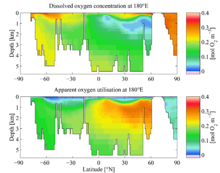 Pacific Ocean sections of dissolved oxygen and apparent oxygen utilisation. Data from the World Ocean Atlas 2005.
Pacific Ocean sections of dissolved oxygen and apparent oxygen utilisation. Data from the World Ocean Atlas 2005.
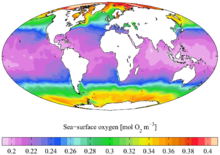
In aquatic environments, oxygen saturation is a relative measure of the amount of oxygen (O2) dissolved in the water. Supersaturation can sometimes be harmful for organisms and cause decompression sickness. Dissolved oxygen (DO) is measured in standard solution units such as millilitres O2 per liter (ml/L), millimoles O2 per liter (mmol/L), milligrams O2 per liter (mg/L) and moles O2 per cubic meter (mol/m3). For example, in freshwater under atmospheric pressure at 20°C, O2 saturation is 9.1 mg/L.
Solubility tables (based upon temperature) and corrections for different salinities and pressures can be found at the USGS web site. Tables such as these of DO in milliliters per liter (ml/L) are based upon empirical equations that have been worked out and tested:[1]
ln(DO) = A1 + A2 * 100 / T + A3 * ln(T / 100) + A4 * T / 100 + S * [B1 + B2 * T / 100 + B3 * (T / 100)2]
Where ln is the natural logarithm and the other variables take the following values:
A1 = -173.4292 B1 = -0.033096 A2 = 249.6339 B2 = 0.014259 A3 = 143.3483 B3 = -0.001700 A4 = -21.8492 T = temperature (kelvin) S = salinity (g/kg) To convert the calculated DO above from ml/L to mg/L, multiply the answer by (P/T)*0.55130, P=mmHg, T=Kelvin
Oxygen content in water can be measured by adding equal quantities of Manganese and Iodine ions in an alkaline solution to a sample of the water. This is then titrated against sodium thiosulfate with a starch indicator and the oxygen concentration determined. One such test is the Winkler test for dissolved oxygen.
See also
References
- ^ Weiss, R. (1970). "The solubility of nitrogen, oxygen, and argon in water and seawater". Deep-Sea Res. 17: 721–35.
Categories:- Hydrology
- Environment stubs
Wikimedia Foundation. 2010.