- Nargenicin
-
Nargenicin 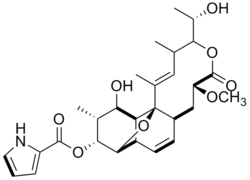
Clinical data Pregnancy cat. ? Legal status ? Pharmacokinetic data Excretion ~ Identifiers CAS number 70695-02-2 ATC code None PubChem CID 6436286 ChemSpider 4885360 
Chemical data Formula C28H37NO8 Mol. mass 515.5953 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Nargenicin is a 28 carbon macrolide with a tricyclic lactone containing a unique ether bridge. The polyketide antibiotic was isolated from Nocardia argentinensis.[1] Nargenicin is effective towards gram-positive bacteria and been shown to have strong antibacterial activity against Staphylococcus aureus, a methicillin resistance bacterium.[2] It has also been shown to induce cell differentiation and be used as a possible treatment for neoplastic diseases.[3]
Biosynthesis
The biosynthesis of nargenicin is believed to be closely related to fatty acid biosynthesis. David E. Cane and colleagues have shown, through feeding experiments, that nargenicin is derived from common precursors acetate and propionate, specifically 4 propionate and 5 acetate building blocks.[4] Experiments using [1-13C]-, [2-13C]-, and [1,2-13C2]acetate and [1-13C]- and [2-13C] propionate established the carbon skeleton of nargenicin. To determine origin of oxygen atoms, [1-18O2, 113C]acetate and [1-18O2,1-13C]propionate where incorporated in the experimental feeding. It was then determined that oxygens located at C-1 and C-11 originate from carboxylate oxygens of the acetate precursors while oxygens at C-9 and C-17 positions originate from propoinate precursors.[4] The rest of the oxygens are believed to originate from molecular oxygen.[5] In a later study, David E. Cane and colleagues incorporated the synthesized deutirated N-acetylcysteamine thioester (2S,3R)-[3-2H, 3-13C]-2-methyl-3-hydroxypentanoic acid into nargenicin.[6] The feeding of the labeled intermediate, and other deuterinated ketide fragments, incorporated in nargenicin provide support of the proposed biosynthesis of the polyketide chain.[7] The following illustrates a proposed biosynthesis of nargenicin.
The polyketide chain produced is electronically capable to go through a lactonization and a diels alder cyclization, as shown below. Experiments have shown the incorporation a complex intermediate of a synthesized pentaketide substrate containing four stereocenters and an E,E-diene into the biosynthesis of nargenicin to result in the labeled macrolide nargenicin.[8] The study provides support for the biosynthetic route of nargenicin and absolute configuration. The last biosynthesis stages include the oxidations at C-2, C-8, C-13 and C-18 positions as well as the attachment of the pyrrole carboxylate.[5]
References
- ^ Celmer WD; Moppett CE; Ware, RS; Watts, PC; Whipple, EB (1980). "Structure of natural antibiotic CP-47,444". J. Am. Chem. Soc. 102 (12): 4203–4209. doi:10.1021/ja00532a036.
- ^ Sohng, K; Yamaguchi, T; Seong, CN; Baik, KS; Park, SC; Lee, HJ (2008). "Production, isolation and biological activity of nargenicin from Nocardia sp. CS682". Arch Pharm Res 31 (10): 1339–1345. doi:10.1007/s12272-001-2115-0. PMID 18958426.
- ^ Kim, SH; Yoo, JC; Kim, TS (2009). "Nargenicin enhances, dihydroxyvitamin D-3 and all-trans retinoic acid-induced leukemia cell differentiation via PKC beta I/MAPK pathways". Biochemical Pharmacology 77 (11): 1694–1701. doi:10.1016/j.bcp.2009.03.004. PMID 19428323.
- ^ a b Cane, DE; Yang, CC (1984). "Biosynthetic Origin of the Carbon Skeleton and Oxygen Atoms of Nargenicin A1". J. Am. Chem. Soc. 106: 784–787. doi:10.1021/ja00315a052.
- ^ a b Cane, DE; Yang, CC (1985). "Nargenicin Biosynthesis: Late Stages Oxidations and Absolute Configuration". J. Antibio. 38: 423–426.
- ^ Cane, DE; Prabhakaran, PC; Tan, W; Ott, WR (1991). "Macrolide Biosynthesis. 6 Mechanism of Polyketide Chain Elongation". Tetrahedron Lett. 32: 5457–5460. doi:10.1016/0040-4039(91)80057-D.
- ^ Cane, DE; Tan, W; Ott, WR (1993). "Nargenicin Biosynthesis. Incorporation of Polyketide Chain Elongation Intermediates and Support for a Proposed Intramolecular Diels-Alder Cyclization". J. Am. Chem. Soc. 115: 527–535. doi:10.1021/ja00055a024.
- ^ Cane, DE; Luo, G (1995). "Biosynthesis of Polyketide Antibiotics. Incorporation of a Pentaketide Chain Elongation Intermediate into Nargenicin". J. Am. Chem. Soc. 117: 6633–6634. doi:10.1021/ja00129a044.
Categories:- Macrolide antibiotics
- Pyrroles
Wikimedia Foundation. 2010.


