- Naphthylvinylpyridine
-
Naphthylvinylpyridine 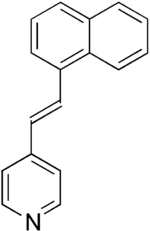 4-[(E)-2-naphthalen-1-ylethenyl]pyridine
4-[(E)-2-naphthalen-1-ylethenyl]pyridineIdentifiers CAS number 16375-56-7 PubChem 5475238 Jmol-3D images Image 1 - C1=CC=C2C(=C1)C=CC=C2/C=C/C3=CC=NC=C3
Properties Molecular formula C17H13N Molar mass 231.29 g/mol  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Naphthylvinylpyridine (NVP) is a naphthalene derivative that possesses anticholinergic activity similar to that of atropine. However, NVP's method of acetylcholine (ACh) antagonism involves inhibiting the enzyme choline acetyltransferase.[1]
Several NVP derivatives have been synthesized and evaluated for their ability to inhibit choline acetyltransferase and protect against nerve toxins.[2][3]
References
- ^ Haubrich, DR; Goldberg, ME (1975). "Homovanillic acid concentration in the rat brain: Effect of a choline acetyltransferase inhibitor and comparison with cholinergic and dopaminergic agents". Neuropharmacology (Squibb Institute for Medical Research) 14 (3): 211–214. doi:10.1016/0028-3908(75)90007-6. PMID 1134625. http://www.sciencedirect.com/science/article/pii/0028390875900076. Retrieved July 2011.
- ^ Cozzari, Costantino; Hartman, BK (1983). "Synthesis of a naphthylvinylpyridine derivative and its use for affinity chromatography of choline acetyltransferase". Analytical Biochemistry (Department of Psychiatry and Neurobiology, Washington University School of Medicine) 133 (1): 120–125. doi:10.1016/0003-2697(83)90231-2. http://www.sciencedirect.com/science/article/pii/0003269783902312. Retrieved July 2011.
- ^ Gray, AP; Henderson, TR (1988). "Approaches to protection against nerve agent poisoning". J Med Chem (Dynamac Corporation) 31 (4): 807–814. PMID 3351860.

This article about an aromatic compound is a stub. You can help Wikipedia by expanding it.
