- Major Histocompatibility Complex and Sexual Selection
-
A pathogen is any agent that causes disease. The body has two defense mechanisms for dealing with pathogenic microorganisms and other harmful substances: the inflammatory reaction and the development of an acquired immunity. Acquired immunity consists of humoral or cell-mediated immunity, and develops after initial contact with a pathogen; it is manifested by the ability of one’s immune system to fight foreign antigens.[1]
All living cells have structures called antigens present on their cell surface. Antigens are either a polysaccharide or a protein that reacts specifically with a cell surface receptor or an antibody. For each person and each type of cell there are different antigens on the cell’s surface. The unique, genetically-determined antigens present on cell surfaces are determined by a cluster of genes on chromosome 6 at loci 6p21.3 called the Major Histocompatibility Complex (MHC).[2] In humans, these antigens were first identified on a type of white blood cell called a leukocyte and were therefore named human leukocyte antigens (HLA antigens). As a result, the human major histocompatibility complex can also be called the HLA system. Often in reference to these antigens and the set of genes responsible for these antigens the terms MHC and HLA are used interchangeably[1]
The antigens on cell surfaces are processed by the cells of the body’s immune system depending on their antigenicity. Specifically, the antigenicity of the HLA surface proteins depends on whether they are one’s own proteins (self-antigens) or whether they are the foreign proteins of another person (non-self-antigens). The HLA proteins on a person’s own cells are recognized by their immune system as such, while non-self-antigens will incite an immune response. Originally, MHC proteins were considered of interest only in regards to organ transplantation because transplants in which the donor and recipient cells contained different MHC proteins resulted in organ rejection unless the immune system was suppressed. Further studies have shown MHC to actually have a much larger role in the immune system than just organ transplantation. The MHC region of genes contains extremely high levels of gene density and diversity; genetic variation within this region plays a vital role in susceptibility to autoimmune, infectious, and other diseases.[2] MHC genes are also involved in various non-immune functions such as olfaction[2] and self/non-self-recognition.[1][3]
Contents
MHC Class I, II, and III Proteins
The surface protein antigens of the Major Histocompatibility Complex system fall into two major classes, called MHC Class I proteins and MHC Class II proteins, as well as an intervening region called MHC Class III proteins.[2] Class I proteins are determined by the HLA-A, HLA-B, and HLA-C genes and are present on nucleated cells and platelets. Class II proteins are determined by HLA-D genes and are integral to the immune system; they are found on lymphocytes, macrophages, and other phagocytic cells whose functions are similar to those of macrophages.[1]
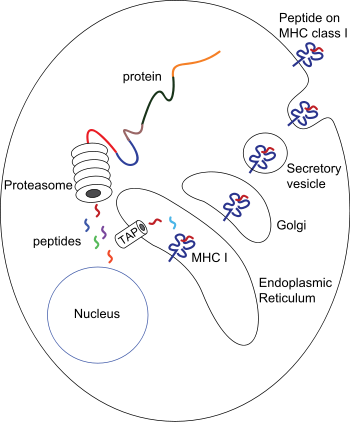 Peptide processing for peptides associated to MHC-I molecules: Proteins present in the cytosol are degraded by the proteasome, and the resulting peptides are internalized by the TAP channel in the endoplasmic reticulum, where they become associated with MHC-I molecules freshly synthesized. The MHC-I/peptide complexes enter in the Golgi apparatus, where they are glycosylated, and from there they enter in secreting vesicles, which fuse with the cell membrane. In this way, the complexes become exposed to the outside of the cell, allowing the contact with circulating T lymphocytes.
Peptide processing for peptides associated to MHC-I molecules: Proteins present in the cytosol are degraded by the proteasome, and the resulting peptides are internalized by the TAP channel in the endoplasmic reticulum, where they become associated with MHC-I molecules freshly synthesized. The MHC-I/peptide complexes enter in the Golgi apparatus, where they are glycosylated, and from there they enter in secreting vesicles, which fuse with the cell membrane. In this way, the complexes become exposed to the outside of the cell, allowing the contact with circulating T lymphocytes.
Studies have demonstrated that genes composing the MHC Class I and Class II regions encode for glycoprotein molecules that are on the cell’s surface; these glycoprotein gene products are directly responsible for recognizing antigens, processing them, and subsequently presenting the processed antigenic peptides to special types of T-cells, CD8+ and CD4+. This is also known as cell-mediated immunity, an important aspect of acquired immunity.[2][4]
Specifically, MHC Class I genes express foreign proteins on the surface of cells that are infected by intracellular pathogens; since each T-cell has its own unique MHC receptor, Cytotoxic T-lymphocytes may bind to the MHC-antigen complex and activate it. This process results in the production of a clone of the particular Cytotoxic T-lymphocyte and the ensuing destruction of any future cell infected with a similar intracellular pathogen. In contrast, MHC Class II genes are responsible for the destruction of extracellular pathogens; these types of pathogens are either phagocytized by macrophages or bound by antibodies. Eventually, they are also presented to T-cells through MHC Class II molecules.[3]
Therefore, through antigen presentation, MHC genes play a vital role in the immune system through both cellular and antibody-mediated defenses. Since MHC genes participate so actively in antigen recognition and processing, the extreme amount of genetic diversity within the MHC therefore enables the immune system to recognize—and process—a broader range of foreign antigens and pathogens; the ability to process more antigens confers the ability to stimulate an immune response towards a wider range of antigens. This is very beneficial for an organism.[3]
Furthermore, genes in the MHC class III region are involved in the regulation of the humoral immune response,[2][5] including aspects of the complement cascade.[2]
For more information on the mechanism of the activation of immune effectors of MHC genes, please refer to Major Histocompatibility Complex.
MHC Evolution and Allelic Diversity
MHC genes, which control the immune response and effective resistance against pathogens, have been able to maintain an extremely high level of allelic diversity throughout time and throughout different populations. In addition to its role in immune function, studies suggest that the MHC is also involved in mate choice for many vertebrates through olfactory cues. There are several proposed hypotheses that address how MHC-associated mating preferences could be adaptive and how the MHC has maintained its enormous allelic diversity.[6][7]
A vast source of genetic variation affecting an organism’s fitness stems from the coevolutionary arms race between hosts and parasites, for which there are two nonmutually exclusive hypotheses: selection for the maintenance of a highly diverse MHC if MHC heterozygotes are more resistant to parasites than homozygotes (heterozygote advantage), or a selection that undergoes a frequency-dependent cycle (Red Queen Hypothesis). If individuals that are heterozygous at the MHC are more resistant to parasites than those who are homozygous, then it would be beneficial for females to choose mates with MHC genes different from their own, also known as disassortative mating. This mechanism of mate choice would result in MHC-heterozygous offspring. Individuals with a heterozygous MHC would be capable of recognizing a wider range of pathogens and therefore of inciting a specific immune response against a greater number of pathogens—thus having an immunity advantage. Unfortunately, the MHC-heterozygote advantage hypothesis has not been adequately tested.[3][7]
The second hypothesis for the maintenance of MHC diversity by parasites is the Red Queen Hypothesis. If individuals’ MHC alleles render different resistances to a particular parasite, then the allele with the highest resistance will be favored, selected for, and consequently spread throughout the population; recombination will have caused the generation of new variants among offspring, which may allow for a quick response to rapidly evolving parasites or pathogens with much shorter generation times. However, if this particular allele becomes common, selection on parasites to avoid recognition by this common allele increases. An advantageous characteristic that allows a parasite to escape recognition will spread and cause selection against what was formerly a resistant allele, enabling the parasite to escape this cycle of frequency-dependent selection; such a cycle will eventually lead to a coevolutionary arms race which may support the maintenance of MHC diversity.[3][7]
 Parasites are in a constant arms race with their host: harvestman suffering from mite pest
Parasites are in a constant arms race with their host: harvestman suffering from mite pest
The Inbreeding Avoidance Hypothesis has less to do with host-parasite relationships than does the Heterozygote Advantage Hypothesis or the Red Queen Hypothesis. The extreme diversity in the MHC would cause individuals sharing MHC alleles to be more likely to be related. As a result, one function of MHC-disassortative mating would be to avoid mating with family members and any harmful genetic consequences that could occur as a result. Mating with relatives, or inbreeding, increases the amount of overall homozygosity—not just locally in the MHC. An increase in genetic homozygosity may be accompanied not only by the expression of recessive diseases and mutations, but by the loss of any potential heterozygote advantage as well.[3][7][8]
In the course of searching for potential mates, it would benefit females to be able to discriminate against “bad” genes in order to increase the health and viability of their offspring. If female mate choice occurs for “good” genes, then it is implied that genetic variation exists among males. Furthermore, one would presume that said difference in genes would impart a difference in fitness as well, which could then potentially be chosen or selected for.[3]
Generally, the extreme polymorphism of MHC genes is believed to be selected for by host-parasite arms races, however disassortative mate choice may maintain genetic diversity in some species. Depending on how parasites alter selection on MHC alleles, MHC-dependent mate-choice may increase the fitness of the offspring by enhancing its immunity, as mentioned earlier. If this is the case, either through the Heterozygote Advantage Hypothesis or the Red Queen Hypothesis, then selection will also favor mating practices that are MHC-dependent.[3]
Therefore, mate choice—with respect to the MHC—has probably evolved so that females choose males either based on diverse genes (Heterozygote Advantage and Inbreeding Avoidance hypotheses) or “good” genes. The fact that females choose is naturally selected, as it would be an advantageous trait for females to be able to choose a male that provided either an indirect or direct benefit. As a result of female choice, sexual selection is imposed on males. This is evidenced by genetic “advertisement.” However, in humans, both sexes exert mate choice. Accordingly, the maintenance of allelic diversity in the MHC would not be due to sexual selection. An example of this would be the existence of exaggerated traits, such as the elaborate tail-feathers of male peacocks.[3]
MHC and Sexual Selection
The Relationship Between Olfaction and MHC
MHC-based sexual selection is known to involve olfactory mechanisms in such vertebrate taxa as fish, mice, humans, primates, birds, and reptiles.[6] At its simplest level, humans have long been acquainted with the sense of olfaction for its use in determining the pleasantness or the unpleasantness of one’s resources, food, etc. At a deeper level, it has been predicted that olfaction serves to personally identify individuals based upon the genes of the MHC.[9]
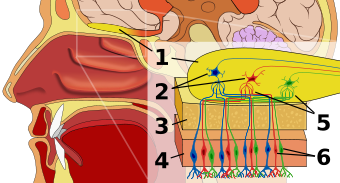 Human olfactory system. 1: Olfactory bulb 2: Mitral cells 3: Bone 4: Nasal epithelium 5: Glomerulus (olfaction) 6: Olfactory receptor cells
Human olfactory system. 1: Olfactory bulb 2: Mitral cells 3: Bone 4: Nasal epithelium 5: Glomerulus (olfaction) 6: Olfactory receptor cells
Chemosensation, which is one of the most primitive senses, has evolved into a specialized sensory system. Humans can not only detect, assess, and respond to environmental (chemical) olfactory cues—especially those used to evoke behavioral and sexual responses from other individuals, also known as pheromones. Pheromones function to communicate one’s species, sex, and perhaps most importantly one’s genetic identity. The genes of the MHC provide the basis from which a set of unique olfactory coding develops.[9]
Although it is not known exactly how MHC-specific odors are recognized, it is currently believed that proteins bound to the peptide-binding groove of the MHC may produce the odorant. Each MHC protein binds to a specific peptide sequence, yielding a set of uniquely bound peptide-MHC complexes for each individual. During cellular turnover, the MHC-peptide complex is shed from the cell surface and the fragments are dispensed in bodily fluids such as blood serum, saliva, and urine. Scientists believe that commensal microflora, microorganisms that line epithelial surfaces open to the external environment such as the gastrointestinal tract and vagina, further degrade these fragments which are then made volatile as a result of this process. Recently, it has been shown that receptors in the vomeronasal organ of mice are activated by peptides having similar characteristics to MHC proteins; further studies may hopefully soon clarify the exact transformation between MHC genotype and an olfactory mechanism.[6][9][10]
MHC-Mediated Mate Choice: Case Studies
Several studies suggest that MHC-related odor preferences and mate choice are demonstrated by humans. However, the role of MHC in human mate choice has been relatively controversial. One study conducted by Ober et al examined HLA types from 400 couples in the Hutterite community and found dramatically fewer HLA matches between husbands and wives than expected when considering the social structure of their community.[11] On the other hand, there was no evidence of MHC-based mate choice in the same study of 200 couples from South Amerindian tribes.[11]
Other studies have approached mate choice based on odor preference. In one study done by Wedekind et al, women were asked to smell male axillary odors collected on T-shirts worn by different males. Women that were ovulating rated the odors of MHC-dissimilar men as more pleasant than those of the MHC-similar men. Furthermore, odors of MHC-dissimilar men often reminded women of current or former partners, suggesting that odor—specifically odor for MHC-dissimilarity—plays a role in mate choice.[12]
In another study done by Wedekind et al, 121 women and men were asked to rank the pleasantness of the odors of sweaty T-shirts. Upon smelling the shirts, it was found that men and women who were reminded of their own mate or ex-mate had dramatically fewer MHC alleles in common with the wearer than would be expected by chance. If the selection for shirts was not random, and actually selected for MHC-dissimilar alleles, this suggests that MHC genetic composition does influence mate choice. Furthermore, when the degree of similarity between the wearer and the smeller was statistically accounted for, there was no longer a significant influence of MHC on odor preference. The results show that MHC similarity or dissimilarity certainly plays a role in mate choice. Specifically, MHC-disassortative mate choice and less similar MHC combinations are selected for.[13]
One interesting aspect of the Wedekind’s experiment was that in contrast to normally cycling women, women taking oral contraceptives preferred odors of MHC-similar men. This would suggest that the pill may interfere with the adaptive preference for dissimilarity.[12][13]
There is also evidence of MHC-associated mate choice in other primates. In the grey mouse lemur Microcebus murinus, there has been shown to be post-copulatory mate-choice that is associated with genetic constitution. Fathers were shown to be more MHC-dissimilar from the mother than were randomly tested males. Additionally, fathers had more differences in amino acid and microsatellite diversity than did randomly tested males. It is hypothesized that this is caused by female cryptic choice.[14]
In mice, both males and females choose MHC-dissimilar partners. It is also known that mice develop the ability to identify family members during early growth and are known to avoid inbreeding with kin, which would support the MHC-mediated mate choice hypothesis for inbreeding avoidance.[7]
Fish are another group of vertebrates shown to display MHC-associated mate choice. Scientists tested the Atlantic salmon, Salmo salar, by observing effects of MHC upon natural spawning salmon that resided in the river versus artificial crosses that were carried out in hatcheries. Logically, the artificial crosses would be bereft of the benefits of mate choice that would naturally be available. The results showed that the offspring of the artificially bred salmon were more infected with parasites: almost four times more than the naturally-spawned offspring were. In addition, wild offspring were more MHC-heterozygous than the artificially-bred offspring. These results support the Heterozygous Advantage hypothesis of sexual selection for MHC-dissimilar mate choice.[15] In another fish, the three-spined stickleback, it has been shown that females desire MHC diversity in their offspring, which affects their mate choice.[16]
Female savannah sparrows, Passerculus sandwichensis, chose MHC-dissimilar males to mate with. It is also known that females are more likely to engage in extra-pair relationships if paired with MHC-similar mates and more dissimilar mates are available. Similarly, MHC diversity in house sparrows, Passer domesticus, suggests that MHC-disassortative mate choice occurs.[7]
MHC-mediated mate choice has also been shown to exist in Swedish sand lizards, Lacerta agilis. Females preferred to associate with odor samples obtained from males more distantly related at the MHC I loci.[17]
Even though many species are socially monogamous, females can accept or actively seek mating outside of the relationship;[18] extra-pair paternity is a mating pattern known to be affiliated with MHC-associated mate choice. Birds are one of the more commonly studied groups of animals to exhibit this sexual behavior. In the scarlet rosefinch Carpocus erythrinus, females engaged in extra-pair paternity much less frequently when their mates were MHC-heterozygous.[19] In the Seychelles warbler Acrocephalus sechellensis, there was no evidence of MHC variation between social mates. However, when females’ social mates were MHC-similar, they were more likely to participate in extra-pair paternity; in most cases, the extra-pair male was significantly more MHC-dissimilar than the social mate.[20]
MHC-mediated mate choice may also occur after copulation at the gametic level, through sperm competition or female cryptic choice. The Atlantic Salmon, Salmo salar, is one species in which sperm competition is influenced by the variation in the Major Histocompatibility Complex, specifically that of the Class I alleles. Atlantic salmon males were found to have higher rates of successful fertilization when competing for eggs from females genetically similar at the class I genes of the MHC.[21]
Another species which exhibits MHC-associated cryptic choice is the Artctic charr Salvelinus alpinus. In this case, however, it seems that sperm selection is more dependent on the ovum. MHC-heterozygous males were found to have significantly more fertilization success than MHC-homozygous males; sperm count, motility, and swimming velocity were not shown to significantly co-vary with similarity or dissimilarity at the MHC. It is proposed that there is a chemo-attraction system responsible for the egg itself being able to discriminate and selectively choose between MHC-heterozygous and MHC-homozygous males.[22]
Contrary to the Atlantic salmon and the Arctic charr, the red junglefowl Gallus gallus is a species in which males instead of females exert cryptic preference. Male junglefowl showed no preference when simultaneously presented with both an MHC-dissimilar and an MHC-similar female. However, they did show a cryptic preference by allocating more sperm to the more MHC-dissimilar of the two.[23]
Male sand lizards Lacerta agilis behave similarly to the male junglefowl. Initial copulation between a male and a female without any rivals was shown to be extended when the male sensed a higher female fecundity. However, second males adjusted the duration of their copulation depending on the relatedness between the female and the first male, believed to be determined by the MHC-odor of the copulatory plug. A closer genetic relatedness between a male and a female sand lizard increased the chances for a successful fertilization and rate of paternity for the second male.[24]
Abortional selection may also be a form of cryptic female choice. Many studies on humans and rodents have found that females may spontaneously abort pregnancies in which the offspring is too MHC-similar. In addition, in vitro fertilizations are more likely to fail when couples have similar MHC genes.[3]
MHC and Sexual Conflict
If males attempt to thwart female mate choice by mating with a female against her will, sexual conflict may interfere with the choice for compatibility at the MHC genes.
In Chinook salmon Oncorhyncus tshawytscha, females were shown to act more aggressively towards MHC-similar males than MHC-dissimilar males, suggesting the presence of female mate choice. Furthermore, males also directed aggression at MHC-similar females. This was accompanied by male harassment of unreceptive females; however, there was a positive correlation between male aggression and reproductive success. The ability of the males to over-power the females’ original mate choice resulted in the offspring of the targets of male aggression having low genetic diversity. Offspring with high genetic diversity seemed to happen only when the operational sex ratio was female-biased, when females were more likely to be able to exert mate choice, and males were less likely to harass females. These results suggest that sexual conflict may interfere with female mate choice for ‘good’ MHC genes.[25]
For more information on sexual conflict, please go to Interlocus Sexual Conflict
References
- ^ a b c d Crowley, Leonard. “Genes of the Histocompatibility Complex.” An Introduction to Human Disease: Pathology and Physiology Correlations. Ed. Amy Bloom. Sudbury: Jones and Bartlett Publishers, 2010. 52-53.
- ^ a b c d e f g Vandiedonck C., K.J.C. The human Major Histocompatibility Complex as a paradigm in genomics research. Briefings in Functional Genomics and Proteomics 8, 379-394(2009).
- ^ a b c d e f g h i j Penn D.J., P.W.K. The evolution of mating preferences and major histocompatibility complex genes. American Naturalist 153, 145-164(1999).
- ^ Benacerraf, B. Role of MHC gene products in immune regulation. Science (New York, N.Y.) 212, 1229-38(1981).
- ^ Vandiedonck, C. et al. Pleiotropic effects of the haplotype patients with autoimmune myasthenia gravis and thymus hyperplasia in. Sciences-New York (2011).
- ^ a b c Milinski M.a Griffiths, S. a c W.K.M. a R.T.B.H. a H.-A.A. b B.T. b Mate choice decisions of stickleback females predictably modified by MHC peptide ligands. Proceedings of the National Academy of Sciences of the United States of America 102, 4414-4418(2005).
- ^ a b c d e f O’Dwyer T.W.a b c Nevitt, G.A. a Individual odor recognition in procellariiform chicks: Potential role for the major histocompatibility complex. Annals of the New York Academy of Sciences 1170, 442-446(2009).
- ^ Westemeier, R. et al. Tracking the long-term decline and recovery of an isolated population. Science (New York, N.Y.) 282, 1695-8(1998).
- ^ a b c Yamazaki K.a Beauchamp, G.K. a S.A. a B.J. b B.E.A. b Odortypes: Their origin and composition. Proceedings of the National Academy of Sciences of the United States of America 96, 1522-1525(1999).
- ^ Bhutta, M.F. Sex and the nose: Human pheromonal responses. Journal of the Royal Society of Medicine 100, 268-274(2007).
- ^ a b Chaix R.a b Cao, C. c D.P. a d Is mate choice in humans MHC-dependent? PLoS Genetics 4, (2008).
- ^ a b Roberts S.C.a c Gosling, L.M. a C.V. b P.M. a MHC-correlated odour preferences in humans and the use of oral contraceptives. Proceedings of the Royal Society B: Biological Sciences 275, 2715-2722(2008).
- ^ a b Wedekind C., F.S. Body odour preferences in men and women: Do they aim for specific MHC combinations or simply heterozygosity? Proceedings of the Royal Society B: Biological Sciences 264, 1471-1479(1997).
- ^ Schwensow N.a b Eberle, M. c S.S. a b d Compatibility counts: MHC-associated mate choice in a wild promiscuous primate. Proceedings of the Royal Society B: Biological Sciences 275, 555-564(2008).
- ^ Consuegra S.a b Garcia De Leaniz, C. a MHC-mediated mate choice increases parasite resistance in salmon. Proceedings of the Royal Society B: Biological Sciences 275, 1397-1403(2008).
- ^ Kurtz J., K.M.A.P.B.H.M.A.W.K.M.R.T.B.H.M.M. Major histocompatibility complex diversity influences parasite resistance and innate immunity in sticklebacks. Proceedings of the Royal Society B: Biological Sciences 271, 197-204(2004).
- ^ Olsson M.a Madsen, T. b c N.J. a W.E. d U.B. a b W.H. b Major histocompatibility complex and mate choice in sand lizards. Proceedings of the Royal Society B: Biological Sciences 270, s254-s256(2003).
- ^ Suter S.M., K.M.F.R.M.D.R. Reed bunting females increase fitness through extra-pair mating with genetically dissimilar males. Proceedings of the Royal Society B: Biological Sciences 274, 2865-2871(2007).
- ^ Promerová M.a Vinkler, M. a b B.J. a P.R. a S.J. b M.P. b A.T. a b Occurrence of extra-pair paternity is connected to social maleʼs MHC-variability in the scarlet rosefinch Carpodacus erythrinus. Journal of Avian Biology 42, 5-10(2011).
- ^ Richardson D.S.a b Komdeur, J. b B.T. c V.S.T. d MHC-based patterns of social and extra-pair mate choice in the Seychelles warbler. Proceedings of the Royal Society B: Biological Sciences 272, 759-767(2005).
- ^ Yeates, S.E. et al. Atlantic salmon eggs favour sperm in competition that have similar major histocompatibility alleles. Proceedings. Biological sciences / The Royal Society 276, 559-66(2009).
- ^ Skarstein F.a Folstad, I. a L.S. a G.M. b MHC and fertilization success in the Arctic charr (Salvelinus alpinus). Behavioral Ecology and Sociobiology 57, 374-380(2005).
- ^ Gillingham M.A.F.a Richardson, D.S. b L.H. a M.A. a c W.K. a b P.T. a Cryptic preference for MHC-dissimilar females in male red junglefowl, Gallus gallus. Proceedings of the Royal Society B: Biological Sciences 276, 1083-1092(2009).
- ^ Olsson, M. et al. Fecundity and MHC affects ejaculation tactics and paternity bias in sand lizards. Evolution; international journal of organic evolution 58, 906-9(2004).
- ^ Sexual-conflict-inhibits-female-mate-choice-for-major-histocompatibility-complex-dissimilarity-in-Chinook-salmon-http-rspb.
See also
Major Histocompatibility Complex
Body Odor and Subconscious Human Sexual Attraction
Categories:- Histochemistry
- Sexual selection
Wikimedia Foundation. 2010.

