- Doyle-Kirmse reaction
-
The Doyle-Kirmse reaction is an organic reaction in which in the original scope an allyl sulfide reacts with trimethylsilyldiazomethane to form the homoallyl sulfide compound.[1] The reaction was first reported by W. Kirmse in 1968 [2] and modified by M.P. Doyle in 1981.[3]
The Kirmse protocol required a copper salt. The reaction type is nucleophilic addition of sulfur to a metal carbene formed from the diazoalkane followed by a Stevens-like rearrangement.
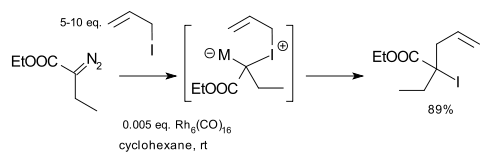
Doyle expanded the scope of the reaction to include diazo compounds such as ethyl diazoacetate, allyl amines and allyl halides with rhodium catalysts such as Hexadecacarbonylhexarhodium. An example is the reaction of ethyl diazoacetate with allyl iodide:
The reaction can also be catalyzed by iron,[4] palladium [5] and silver [6] Modifications using other carbenes are reported e.g. (2-furyl)carbenoids.[7] With use of certain propargyl sulfides the reaction product is an allene.[6][8]References
- ^ Name reactions and reagents in organic synthesis Bradford P. Mundy,Michael G. Ellerd,Frank G. Favaloro
- ^ Kirmse, W. and Kapps, M. (1968), Reaktionen des Diazomethans mit Diallylsulfid und Allyläthern unter Kupfersalz-Katalyse. Chemische Berichte, 101: 994–1003. doi:10.1002/cber.19681010333
- ^ Highly effective catalytic methods for ylide generation from diazo compounds. Mechanism of the rhodium- and copper-catalyzed reactions with allylic compounds Michael P. Doyle, William H. Tamblyn, Vahid Bagheri J. Org. Chem., 1981, 46 (25), pp 5094–5102 doi:10.1021/jo00338a008
- ^ Iron-Catalyzed Doyle−Kirmse Reaction of Allyl Sulfides with (Trimethylsilyl)diazomethane David S. Carter and David L. Van Vranken Org. Lett., 2000, 2 (9), pp 1303–1305 doi:10.1021/ol005740r
- ^ Palladium-catalyzed insertion reactions of trimethylsilyldiazomethane Kevin L. Greenman, David S. Carter and David L. Van Vranken Tetrahedron Volume 57, Issue 24, 11 June 2001, Pages 5219-5225 doi:10.1016/S0040-4020(01)00363-5
- ^ a b Silver-catalysed Doyle–Kirmse reaction of allyl and propargyl sulfides Paul W. Davies, Sébastien J.-C. Albrecht and Giulio Assanelli Org. Biomol. Chem., 2009, 7, 1276-1279 doi:10.1039/B822584B
- ^ Doyle−Kirmse Reaction of Allylic Sulfides with Diazoalkane-Free (2-Furyl)carbenoid Transfer Yumiko Kato,, Koji Miki,, Fumiaki Nishino,, Kouichi Ohe, and, Sakae Uemura Organic Letters 2003 5 (15), 2619-2621 doi:10.1021/ol034731q
- ^ Iron-Catalyzed Reaction of Propargyl Sulfides and Trimethylsilyldiazomethane Rosalind Prabharasuth and, David L. Van Vranken The Journal of Organic Chemistry 2001 66 (15), 5256-5258 doi:10.1021/jo010247u
Categories:
Wikimedia Foundation. 2010.