- Darusentan
-
Darusentan 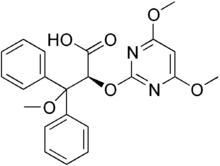
Systematic (IUPAC) name (2S)-2-(4,6-Dimethoxypyrimidin-2-yl)oxy-3-methoxy-3,3-di(phenyl)propanoic acid Clinical data Pregnancy cat. ? Legal status ? Routes Oral Pharmacokinetic data Metabolism Hepatic Half-life 12.5 hours Identifiers CAS number 171714-84-4 
ATC code None PubChem CID 177236 UNII 33JD57L6RW 
ChEMBL CHEMBL23261 
Chemical data Formula C22H22N2O6 Mol. mass 410.42 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Darusentan (LU-135252; HMR-4005) is a endothelin receptor antagonist. Gilead Colorado, a subsidiary of Gilead Sciences,[1] under license from Abbott Laboratories, is developing darusentan for the potential treatment of uncontrolled hypertension.
In June 2003, Myogen licensed the compound from Abbott for its application in the cancer field.[2]
In May 2007, a randomized, double-blind, active control, parallel assignment, safety and efficacy phase III trial was initiated in subjects who had completed the maintenance period of the DAR-312 study.
References

This pharmacology-related article is a stub. You can help Wikipedia by expanding it.
