- Cyanate hydratase
-
Cyanate_lyase 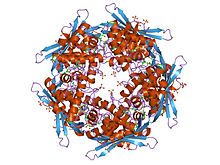
structure of cyanase with the di-anion oxalate bound at the enzyme active site Identifiers Symbol Cyanate_lyase Pfam PF02560 InterPro IPR003712 SCOP 1dw9 Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary In molecular biology, cyanate hydratase EC 4.2.1.104 is an enzyme which catalyses the hydrolysis of cyanate. It is also known as cyanate lyase or cyanase.
Some bacteria can overcome the toxicity of environmental cyanate by hydrolysis of cyanate by this enzyme.[1] Cyanate hydratase is found in bacteria and plants and catalyses the reaction of cyanate with bicarbonate to produce ammonia and carbon dioxide. The cyanate hydratase monomer is composed of two domains. The N-terminal domain shows structural similarity to the DNA-binding alpha-helix bundle motif. The C-terminal domain has an 'open fold' with no structural homology to other proteins. The dimer structure reveals the C-terminal domains to be intertwined, and a decamer is formed by a pentamer of these dimers. The active site of the enzyme is located between dimers and is composed of residues from four adjacent subunits of the homodecamer.[2]
References
- ^ Sung YC, Fuchs JA (October 1988). "Characterization of the cyn operon in Escherichia coli K12". J. Biol. Chem. 263 (29): 14769–75. PMID 3049588.
- ^ Walsh MA, Otwinowski Z, Perrakis A, Anderson PM, Joachimiak A (May 2000). "Structure of cyanase reveals that a novel dimeric and decameric arrangement of subunits is required for formation of the enzyme active site". Structure 8 (5): 505–14. doi:10.1016/S0969-2126(00)00134-9. PMID 10801492.
This article includes text from the public domain Pfam and InterPro IPR003712
Categories:- Protein domains
Wikimedia Foundation. 2010.
