- Salcomine
-
Salcomine[1] 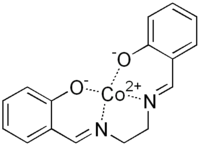 Other namesN,N′-Bis(salicylidene)ethylenediaminocobalt(II)
Other namesN,N′-Bis(salicylidene)ethylenediaminocobalt(II)Identifiers CAS number 14167-18-1 
PubChem 11012797 Jmol-3D images Image 1 - C1=CC(=CNCCNC=C2C=CC=CC2=O)C(=O)C=C1.[Co]
Properties Molecular formula C16H14CoN2O2 Molar mass 325.23 g mol−1 Hazards MSDS Oxford MSDS R-phrases R36/37/38 S-phrases S26 S36  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Salcomine is a coordination complex derived from the salen ligand and cobalt. The complex, which is planar, and a variety of its derivatives are carriers for O2 as well as oxidation catalysts.[2]
Preparation and structure
The compound features square planar Co(II). It forms a variety of five-coordinate adducts, as is typical for complexes of d7 metals. In addition to oxygen and nitrogen donor sites provided by the salen2-, the metal typically is bound to pyridine (py) to give the complex Co(salen)(py). Such complexes bind O2 to give derivatives of the type (μ-O2)[Co(salen)py]2 and [Co(salen)py(O2)].[2]
Salcomine is commercially available. It may be easily synthesized from cobalt(II) acetate and salenH2:[3]
Applications
The 1938 report that this compound reversibly bound O2 led to intensive research on salen and related ligands for the storage or transport of oxygen.[4] Salcomine catalyzes the oxidation of 2,6-disubstituted phenols by dioxygen.[5]
References
- ^ N,N′-Bis(salicylidene)ethylenediaminocobalt(II) at Sigma-Aldrich
- ^ a b Shoichiro Yamada “Advancement in stereochemical aspects of Schiff base metal complexes” Coordination Chemistry Reviews 1999, volume 190–192, 537–555.
- ^ Appleton, T. G. (1977). "Oxygen Uptake by a Cobalt(II) Complex". J. Chem. Ed. 54 (7): 443. doi:10.1021/ed054p443.
- ^ Tokuichi Tsumaki (1938). "Nebenvalenzringverbindungen. IV. Über einige innerkomplexe Kobaltsalze der Oxyaldimine". Bulletin of the Chemical Society of Japan 13 (2): 252–260. doi:10.1246/bcsj.13.252.
- ^ C. R. H. I. De Jonge, H. J. Hageman, G. Hoentjen, and W. J. Mijs (1988), "Oxidation with Bis(Salicylidene)ethylenediiminocobalt(II) (Salcomine): 2,6-Di-''tert''-butyl-''p''-benzoquinone", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv6p0412; Coll. Vol. 6: 412
Categories:- Metal salen complexes
- Cobalt compounds
Wikimedia Foundation. 2010.
