- Cholesterol-dependent cytolysin
-
Cholesterol-Dependent Cytolysins (CDC) are a family of β-barrel pore-forming exotoxins that are secreted by Gram-positive bacteria. CDC are secreted as water-soluble monomers of 50-70 kDa, that once bound to the target cell, will form a circular homo-oligomeric complex containing up to 50 monomers.[1] Through multiple conformational changes, the β-barrel transmembrane structure (~250Ǻ in diameter depending on the toxin) is formed and inserted into the target cell membrane. The presence of cholesterol in the target membrane is required for pore formation, though presence of cholesterol is not required by all CDC for binding. Intermedilysin (ILY) secreted by Streptococcus intermedius will bind only to target membranes containing a specific protein receptor, but cholesterol is required by intermedilysin (ILY) for pore formation. The distribution of cholesterol in the membrane can affect toxin binding, though the exact molecular mechanism that cholesterol regulates the cytolytic activity of the CDC is not totally understood.
Contents
Cyto-lethal effects
Once the pore is formed within the target cell membrane, the regulation of the intracellular environment and what enters and leaves the cell is lost. The pore being ~250Ǻ in diameter is large enough to allow the loss of amino acids, nucleotides, small proteins as well as ions (Ca2+,Na+, K+, etc.). The loss of calcium in particular, which is involved in multiple molecular pathways will have a large impact on cell survival. The pore will also lead to an influx of water, which may lead to blebbing and cell death.
Purpose
Bacteria invest energy into creating these toxins because they act as virulence factors.[1] By targeting immune cells such as macrophages the bacteria will be protected against phagocytosis and destruction by respiratory burst.[2]
Structure
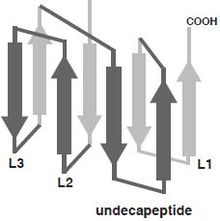
The 28 CDC family members are known to vary in amino acid identity from 28.1 – 99.6%, with amino acid sequences ranging from 471 to 665 amino acids.[3] Within the primary structure of the proteins there is a low degree of conservation at the N-terminus, it is presumed because some CDC contain additional domains at this region and different species use different signal sequences for secretion.[4] The CDC monomer consist of 4 structural domains, with domain 4 (D4) being involved with membrane binding.[5] Multiple CDC monomers will oligomerize once bound to the target cell membrane forming a β-barrel structure which will be inserted into the target cell membrane. The core section of amino acids, which are required for pore formation are more conserved between CDC, which allows all CDC to exhibit similar three-dimensional structures and function. The structurally conserved domain 4 of CDC contains four conserve loops L1-L3 and an undecapeptide region, which is believed to be involved in cholesterol dependent recognition.[6] Single amino acid modifications in these loops prevented Perfingolysin O (PFO), which is a CDC secreted by Clostridium perfringens from binding to cholesterol rich liposomes.[3]
Domain 4 of Perfringolysin O with labeled loops L1, L2, L3, and Undecapeptide region.[3]
Assembly/Translocation
The mechanism of pore formation of perfingolysin O (PFO), which is secreted by Clostridium perfringens, begins with encountering and binding to cholesterol on the target membrane. The C-terminus of PFO domain 4 (D4) encounters the membrane first. The binding of D4 triggers a structural rearrangement in which the PFO monomers oligomerize forming the pre-pore complex.[1][7]
EM reconstruction of Perfringolysin O pre-pore(A) and pore(B) structure.[8]
The binding of CDC to the target membrane is required for oligmerization.[1] The oligomerization of the CDC requires the exposure of hidden polypeptide regions that are triggered by conformational changes induced by protein-lipid interactions or protein-protein interactions.[1] The water-soluble form of the toxins is prevented from oligomerizing by having the access of one edge of a core β-sheet in the monomer blocked. To be specific, β5, a short polypeptide loop, hydrogen-bonds to β4, preventing β4 interaction with β1 on the adjacent monomer. The binding of D4 to the membrane surface triggers a conformational change in domain 3, which rotates β5 away from β4, exposing β4 allowing it to interact with the β1 strand of another PFO molecule, initiating oligomerization.
Water soluble monomeric form of perfingolysin O (PFO) domain 3 showing β5 loop bound to β4 preventing premature oligomerization.[1]
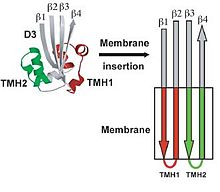
Unlike most of the exposed surface residues of CDC that are not conserved the residues at the surface of the D4 tip, which are involved in membrane interactions, are highly conserved.[3] Pore formation begins once two amphipathic transmembrane β-hairpins from ~35 PFO monomers are concertedly insert, which then create a large β-barrel that perforates the membrane.[7] The toxin gets around the energy barrier of inserting the CDC into the membrane by the formation of the β-barrel, which will lower the energy requirements compared to what would be required for the insertion of single β-hairpins. In the water-soluble monomeric form of CDC, the transmembrane β-hairpins that are located on both sides of the central β-sheet on domain 3 are each folded as three short α-helices to minimize the exposure of hydrophobic residues.[1] The α-helices are inserted into the target cell membrane bilayer and a conformational change takes place into amphipathic β-hairpins. A concerted mechanism of insertion is required so that the hydrophilic surfaces of the β-hairpins remain exposed to the aqueous medium, and not the hydrophobic membrane core.
Ribbon representation of water soluble monomeric form of perfingolysin O (PFO) with labeled domains.
Six short α-helices in D3 unfold to form two transmembrane β-hairpin (TMH), TMH1 (red) and TMH2 (green).[5]
Specificity
The binding of CDC to its target membrane requires the recognition of cholesterol or in the case of intermedilysin (ILY), the recognition of CD59 membrane-anchored protein. The recognition of cholesterol provides specificity for eukaryotic cells and the specificity for the glycosylphosphatidylinositol-anchored protein CD59 provides specificity for human cells. Even though cholesterol is not required for intermedilysin (ILY) to bind to a target cell, the presence of cholesterol is required for pore formation by all CDC.[9]
CDC are sensitive to both oxygen and cholesterol. Toxins isolated-form culture supernatants were inactivated once exposed to oxygen after being pre-incubated with cholesterol.[10] CDC are also pH-sensitive. A change of pH in a medium from 7.4 to 6.0 caused a conformational change in perfringolysin O, leading to the exposure of tryptophan residues to the aqueous solvent and altering the minimum cholesterol threshold required for binding.[11] Another CDC, listeriolysin O (LLO), which optimally functions at an acidic pH, will lose its function at a neutral pH. Listeriolysin O function can be restored if the cholesterol concentration within the target membrane is increased.[3]
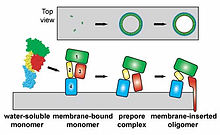 Stages of insertion or Perfingolysin O monomers and oligomerization in target cell membrane.[12]
Stages of insertion or Perfingolysin O monomers and oligomerization in target cell membrane.[12]
Role of cholesterol
The presents of cholesterol in the membrane of the target cell is required for CDC pore formation. The arrangement of cholesterol molecules in the bilayer is important for successful binding. The non-polar hydrocarbon tail of cholesterol orients itself toward the polar center of the membrane lipid bilayer, while the 3-β-OH group is oriented closer to the ester bonds formed by the fatty acid chains, and glycerol backbones closer to the membrane surface. Even with the 3-β-OH group near the membrane surface, it is not very exposed compared to the phospholipid head groups. The availability of cholesterol at the membrane surface is dependent upon its interaction with other membrane components such as phospholipids and proteins; and the more cholesterol interacts with these components the less available it is to interact with extramembranous molecules. Some factors that affect cholesterols availability are size of the polar head groups and the ability of the phospholipid to hydrogen bond with the 3-β-OH group of cholesterol.[13] Cholesterol associates with phospholipids, forming a stoichiometric complex, though, increasing the cholesterol beyond a certain point, free cholesterol will begin to precipitate out of the membrane. The binding and pore formation of CDC will occur when the concentration of cholesterol exceeds the association capacity of the phospholipids, allowing the excess cholesterol to associate with the toxin. The presence of cholesterol aggregates in an aqueous solution were sufficient to initiate a conformation change and oligomerization of perfringolysin O (PFO), while no changes were seen by perfingolysin O with epicholesterol aggregates in solution.[14] Epicholesterol is a sterol that differs from cholesterol by the orientation of the 3-β-OH group, which is axial in epicholesterol and equatorial in cholesterol. Since the orientation of the hydroxyl group has such an effect on the bind/pore-formation of CDC, the equatorial conformation may be required for docking of the sterol to the binding pocket in domain 4, or to be properly exposed at the surface of lipid structures.
Effects of other membrane lipids
The phospholipid composition of a cell membrane affects the arrangement of cholesterol within the membrane and the ability for CDC to bind and initiate pore-formation. For example, perfringolysin O will preferentially bind to cholesterol-rich membranes composed mainly of phospholipids containing 18-carbon acyl chains.[13] Lipids having a conical molecular shape alter the energetic state of membrane cholesterol, augmenting the interaction of the sterol with the cholesterol-specific cytolysin.[15] Since high cholesterol concentrations are required for CDC binding/pore-formation, it was thought that CDC would associate with lipid rafts. A later study showed that sphingomyelin, a necessary component of lipid raft formation, inhibited rather than promoted the binding of perfringolysin O to the target membrane.[16]
Possible Coordination With Other Toxins
It is possible that the exposure of cholesterol at the membrane surface might be facilitated by other membrane-damaging toxins secreted such as phospholipase C, which cleave the head groups of phospholipids increasing the exposure of cholesterol. This has been seen in two organisms, perfingolysin O (CDC) and α-toxin secreted by clostridial myonecrosis.[17] and listeriolysin O (CDC) and phospholipases C released by Listeria monocytogenes leading to the virulence of these bacteria.[18]
See also
- Exotoxin
- Pore-Forming Toxin
References
- ^ a b c d e f g Ramachandran, R., Tweten, R. K. & Johnson, A. E. (2004) Membrane-dependent conformational changes initiate cholesterol-dependent cytolysin oligomerization and intersubunit β-strand alignment. Nat. Struct. Mol. Biol., 11, 697-705.
- ^ Alberts, Bruce. Molecular Biology of the Cell. 5th ed. New York: Garland Science, 2008. Print
- ^ a b c d e Heuck, Alejandro P., Paul C. Moe, and Benjamin B. Johnson. "The Cholesterol-dependent Cytolysin Family of Gram-positive Bacterial Toxins." Subcell Biochem 51 (2010): 551-77. National Center for Biotechnology Information. Web. 15 Mar. 2011.
- ^ Farrand, S., Hotze, E., Friese, P., Hollingshead, S. K., Smith, D. F., Cummings, R. D.,Dale, G. L. & Tweten, R. K. (2008) Characterization of a streptococcal cholesteroldependent cytolysin with a Lewis y and b Specific Lectin Domain. Biochemistry, 47, 7097-7107.
- ^ a b Ramachandran, R., Heuck, A. P., Tweten, R. K. & Johnson, A. E. (2002) Structural insights into the membrane-anchoring mechanism of a cholesterol-dependent cytolysin. Nat. Struct. Mol. Biol., 9, 823-827.
- ^ Soltani, C. E., Hotze, E. M., Johnson, A. E. & Tweten, R. K. (2007b) Structural elements of the cholesterol-dependent cytolysins that are responsible for their cholesterol-sensitive membrane interactions. Proc. Natl. Acad. Sci. U.S.A., 104, 20226-20231.
- ^ a b Dang, T. X., Hotze, E. M., Rouiller, I., Tweten, R. K. & Wilson-Kubalek, E. M. (2005) Prepore to pore transition of a cholesterol-dependent cytolysin visualized by electron microscopy. J. Struct. Biol., 150, 100-108.
- ^ Tilley, Sarah J., Elena V. Orlova, Robert J.C. Gilbert, Peter W. Andrew, and Helen R. Saibil. "Structural Basis of Pore Formation by the Bacterial Toxin Pneumolysin." Cell 121.2 (2005): 247-56. Cell. 22 Apr. 2005. Web. 20 Apr. 2011
- ^ Giddings, K. S., Johnson, A. E. & Tweten, R. K. (2003) Redefining cholesterol's role in the mechanism of the cholesterol-dependent cytolysins. Proc. Natl. Acad. Sci. U.S.A., 100, 11315-11320
- ^ Alouf, J. E., Billington, S. J. & Jost, B. H. (2006) Repertoire and general features of the family of cholesterol-dependent cytolysins. In Alouf, J. E. & Popoff, M. R. (Eds.) The Comprehensive Sourcebook of Bacterial Protein Toxins. 3rd ed., pp. 643-658, Oxford, England. Academic Press
- ^ Nelson, L. D., Johnson, A. E. & London, E. (2008) How interaction of perfringolysin O with membranes is controlled by sterol structure, lipid structure, and physiological low pH: insights into the origin of perfringolysin O-lipid raft interaction J. Biol. Chem., 283, 4632-4642.
- ^ Heuck, Alejandro. "Dr. Alejandro Heuck." Index of /. Web. 20 Apr. 2011. <http://people.biochem.umass.edu/aheuck/WebPage/aph.htm>.
- ^ a b Ohno-Iwashita, Y., Iwamoto, M., Ando, S. & Iwashita, S. (1992) Effect of lipidic factors on membrane cholesterol topology - mode of binding of θ-toxin to cholesterol in liposomes. Biochimica et Biophysica Acta, 1109, 81-90.
- ^ Heuck, A. P., Savva, C. G., Holzenburg, A. & Johnson, A. E. (2007) Conformational changes that effect oligomerization and initiate pore formation are triggered throughout perfringolysin O upon binding to cholesterol. J. Biol. Chem., 282, 22629-22637
- ^ Zitzer, A., Westover, E. J., Covey, D. F. & Palmer, M. (2003) Differential interaction of the two cholesterol-dependent, membrane-damaging toxins, streptolysin O and Vibrio cholerae cytolysin, with enantiomeric cholesterol. FEBS Lett., 553, 229-231.
- ^ Flanagan, J. J., Tweten, R. K., Johnson, A. E. & Heuck, A. P. (2009) Cholesterol exposure at the membrane surface is necessary and sufficient to trigger perfringolysin O binding. Biochemistry, 48, 3977-3987.
- ^ Awad, M. M., Ellemor, D. M., Boyd, R. L., Emmins, J. J. & Rood, J. I. (2001) Synergistic effects of alpha-toxin and perfringolysin O in Clostridium perfringens-mediated gas gangrene. Infect. Immun., 69, 7904-7910.
- ^ Alberti-Segui, C., Goeden, K. R. & Higgins, D. E. (2007) Differential function of Listeria monocytogenes listeriolysin O and phospholipases C in vacuolar dissolution following cell-to-cell spread. Cell. Microbiol., 9, 179-195.
Categories:- Bacterial toxins
Wikimedia Foundation. 2010.