- Cascade reaction
-
A cascade reaction or tandem reaction or domino reaction is a consecutive series of intramolecular organic reactions which often proceed via highly reactive intermediates. It allows the organic synthesis of complex multinuclear molecules from a single acyclic precursor. The substrate contains many functional groups that take part in chemical transformations one at the time. Often a functional group is generated in situ from the previous chemical transformation. The definition includes the prerequisite intramolecular in order to distinguish this reaction type from a multi-component reaction. In this sense it differs from the definition of a biochemical cascade. The main advantages of a cascade reaction in organic synthesis are that the reaction is often fast due to its intramolecular nature, the reaction is also clean, displays high atom economy and does not involve workup and isolation of many intermediates.
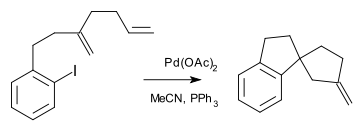
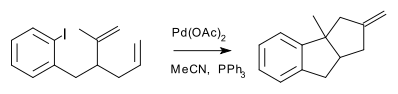
A cascade reaction is sometimes called a living reaction because it shares some characteristics with a living polymerization. In cascade reactions one can identify an initiation site, a relay moiety and a termination moiety. Examples of cascade reactions are numerous (e.g. the Aldol-Tishchenko reaction) and especially so in alkyne chemistry (the Banert cascade to name just one) or polyolefin polycycloisomerizations. Other alkyne coupling reactions are classified based on common features such as type of compound synthesised, for instance the spiro mode cascade [1]:
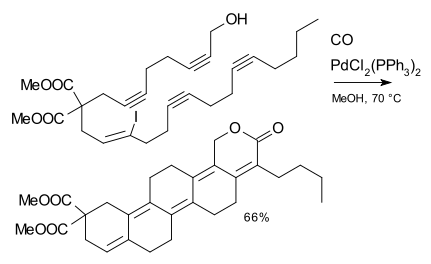
or the linear-fused mode cascade,[2] through application of the intramolecular Heck reaction:
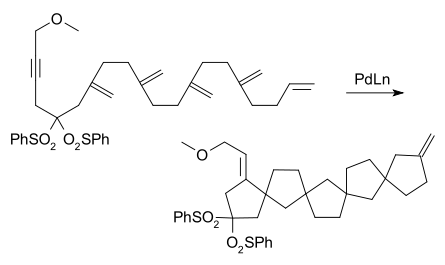
or the zipper mode cascade.[3]
Other cascade reactions are included in Diels-Alder reactions, oxirane ring-opening reactions,[4][5][6] and Pauson–Khand reactions.[7]
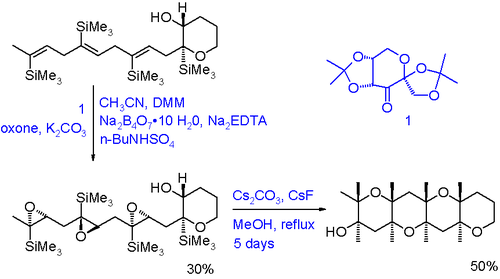
An example of an oxirane cascade reaction is given by the synthesis of certain polyether ladder polymers [8]:
This type of ladder compounds are found in marine lifeforms such as red tide. The tri-epoxide is prepared from a triene through asymmetric Shi epoxidation and oxone as primary oxidizing agent. The hydroxyl group in the tri-epoxide is activated as a nucleophile by the presence of the base caesium carbonate The bulky trimethylsilyl groups make such that the polyether is formed with the correct stereochemistry and they are removed in situ by caesium fluoride.
References
- ^ Barry M. Trost and Yian Shi (1991). "A Pd-Catalyzed Zipper Reaction". J. Am. Chem. Soc. 113 (2): 701–703. doi:10.1021/ja00002a064.
- ^ M. M. Abelman and L. E. Overman (1988). "Palladium-catalyzed polyene cyclizations of dienyl aryl iodides". J. Am. Chem. Soc. 110 (7): 2328–2329. doi:10.1021/ja00215a068.
- ^ T. Sugihara, C. Coperet, Z. Owczarczyk, L. S. Harring and E.-i. Negishi (1994). "Deferred Carbonylative Esterification in the Pd-Catalyzed Cyclic Carbometalation-Carbonylation Cascade". J. Am. Chem. Soc. 116 (17): 7923–7924. doi:10.1021/ja00096a070.
- ^ Ronald Grigg and Visuvanathar Sridharan (1998). "Heterocycles via Pd catalysed molecular queuing processes. Relay switches and the maximisation of molecular com plexity". Pure Appl. Chem. 70 (5): 1047–1057. doi:10.1351/pac199870051047. http://www.iupac.org/publications/pac/1998/pdf/7005x1047.pdf.
- ^ Tandem Reaction Sequences Chris Borths MacMillan Group Meeting November 21, 2000 article
- ^ Xiaomin Jin Cook Group Feb. 11, 2005 Article
- ^ Nakcheol Jeong, Byung Ki Sung, Jin Sung Kim, Soon Bong Park, Sung Deok Seo, Jin Young Shin, Kyu Yeol In, and Yoon Kyung Choi (2002). "Pauson–Khand-type reaction mediated by Rh(I) catalysts". Pure Appl. Chem. 74 (1): 85–91. doi:10.1351/pac200274010085. http://www.iupac.org/publications/pac/2002/pdf/7401x0085.pdf.
- ^ Graham L. Simpson, Timothy P. Heffron, Estíbaliz Merino, and Timothy F. Jamison (2006). "Ladder Polyether Synthesis via Epoxide-Opening Cascades Using a Disappearing Directing Group". J. Am. Chem. Soc. 128 (4): 1056–1057. doi:10.1021/ja057973p. PMID 16433504.
Categories:
Wikimedia Foundation. 2010.