Trigonal bipyramidal molecular geometry
- Trigonal bipyramidal molecular geometry
Infobox molecular geometry
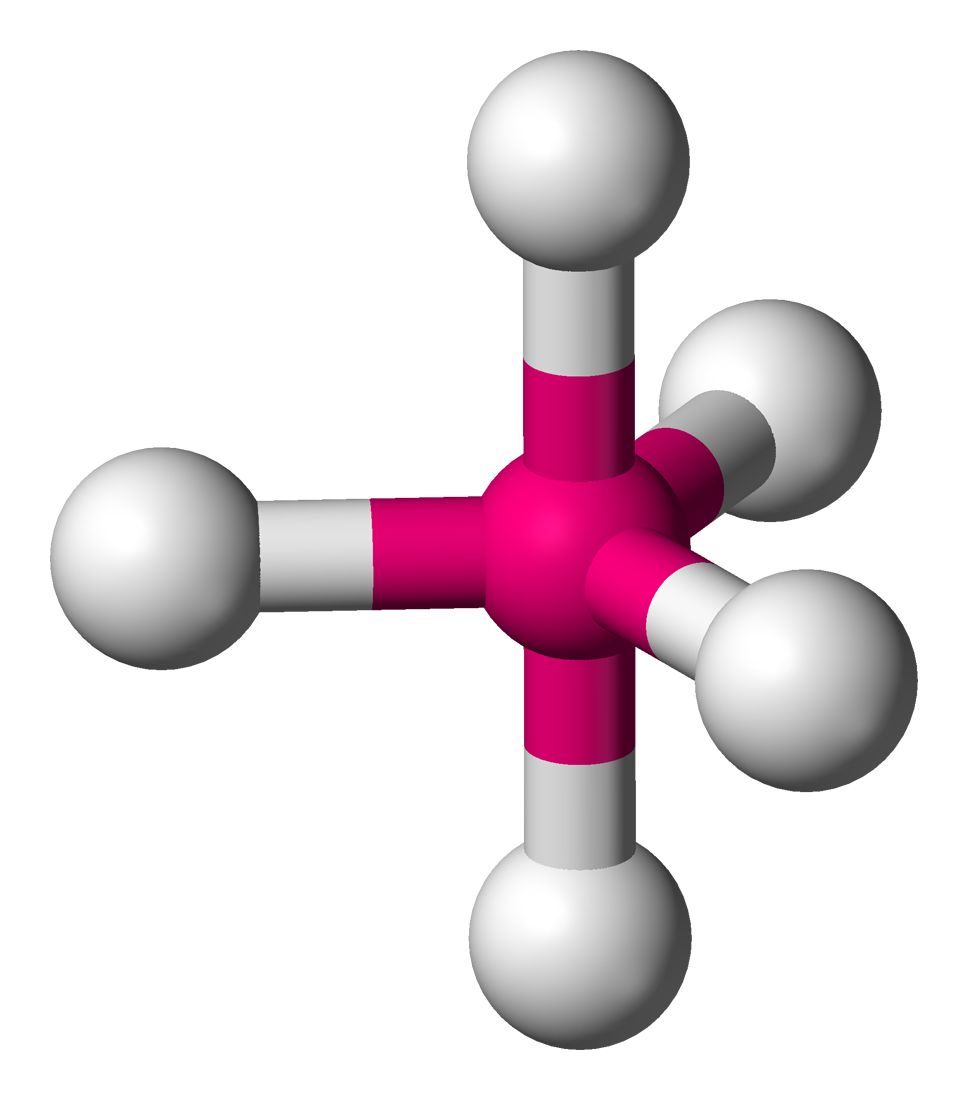
Symmetry_group= D3h
Electron_direction=5
Atom_direction=5
Bond_angle=90°, 120°
mu= 0
Examples=PF5
In chemistry a trigonal bipyramid formation is a molecular geometry with one atom at the center and 5 more atoms at the corners of a triangular dipyramid. This is one of the only cases where bond angles surrounding an atom are not identical (see also pentagonal dipyramid), which is simply because there is no geometrical arrangement which can result in five equally sized bond angles in three dimensions.
Behavior
Isomers with a trigonal bipyramidal geometry are able to interconvert through a process known as Berry pseudorotation. Pseudorotation is similar in concept to the movement of a conformational diastereomer, though no full revolutions are completed. In the process of pseudorotation, two equatorial ligands (both of which have a shorter bond length than the third) "shift" toward the molecule's axis, while the axial ligands simultaneously "shift" toward the equator, creating a constant cyclical movement. Pseudorotation is particularly notable in simple molecules such as PF5.
Examples
Phosphorus pentachloride is a molecule with a trigonal bipyramidal geometry. The phosphorus atom shares a plane with three chlorine atoms which are at 120 degrees angles to each other ("equatorial" positions), with two more chlorine atoms above and below the plane ("apical" or "axial" positions). The triiodide ion is also based upon a trigonal bipyramid, but with the equatorial positions filled with lone pairs of electrons. In phosphorus compounds with mixed substituents apicophilicity is observed.
ee also
*AXE method
*Molecular geometry
External links
* [http://www.3dchem.com/3D Chem| Chemistry, Structures, and 3D Molecules]
* [http://www.iumsc.indiana.edu/IUMSC| Indiana University Molecular Structure Center]
* [http://www.phys.ncl.ac.uk/staff/njpg/symmetry/Molecules_l3d.html Point Group Symmetry| Point Group Symmetry Interactive Examples]
* [http://chemlab.truman.edu/CHEM121Labs/MolecularModeling1.htm| Molecular Modeling]
* [http://intro.chem.okstate.edu/1314F97/Chapter9/3BP.html| Animated Trigonal Planar Visual]
Wikimedia Foundation.
2010.
Look at other dictionaries:
Molecular geometry — Geometry of the water molecule Molecular geometry or molecular structure is the three dimensional arrangement of the atoms that constitute a molecule. It determines several properties of a substance including its reactivity, polarity, phase of… … Wikipedia
Square pyramidal molecular geometry — In molecular geometry, square based pyramidal geometry describes the shape of certain compounds with the formula ML5 where L is a ligand. If the ligand atoms were connected, the resulting shape would be that of a pyramid with a square base. The… … Wikipedia
Octahedral molecular geometry — Idealized structure of a compound with octahedral coordination geometry. In chemistry, octahedral molecular geometry describes the shape of compounds where in six atoms or groups of atoms or ligands are symmetrically arranged around a central… … Wikipedia
Seesaw molecular geometry — Infobox molecular geometry Symmetry group= C2V Electron direction=5 Atom direction=4 Bond angle=90°, 120°, 180° mu= >0 Examples= SF4 Seesaw is a type of molecular geometry where the central atom has one lone pair of electrons, and there are four… … Wikipedia
Molecular symmetry — in chemistry describes the symmetry present in molecules and the classification of molecules according to their symmetry. Molecular symmetry is a fundamental concept in chemistry, as it can predict or explain many of a molecule s chemical… … Wikipedia
Coordination geometry — The term coordination geometry is used in a number of related fields of chemistry and solid state chemistry/physics. Contents 1 Molecules 2 Inorganic coordination complexes 3 Crystallography usage 4 … Wikipedia
Antimony — This article is about the element. For the town, see Antimony, Utah. Not to be confused with Antinomy, a type of paradox. tin ← antimony → tellurium As ↑ Sb ↓ Bi … Wikipedia
Van der Waals strain — In chemistry, van der Waals strain is strain resulting from van der Waals repulsion when two substituents in a molecule approach each other with a distance less than the sum of their van der Waals radii. Van der Waals strain is also called van… … Wikipedia
Dicarbonyltris(triphenylphosphine)ruthenium(0) — IUPAC name (TBPY 5 22) dicarbonyltris … Wikipedia
Phosphorane — A phosphorane is a functional group in chemistry with pentavalent phosphorus. It has the general structure PR5. The parent compound is the non stable phosphoran PH5 or λ5 Phosphan (lambda5phosphan) according to IUPAC nomenclature. In the same… … Wikipedia

