- Doebner reaction
-
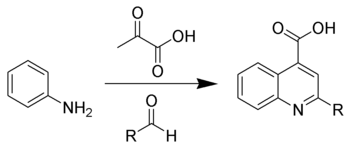
The Doebner reaction is the chemical reaction of an aniline with an aldehyde and pyruvic acid to form quinoline-4-carboxylic acids.[1][2]
References
See also
- Conrad-Limpach reaction
- Doebner-Miller reaction

This chemical reaction article is a stub. You can help Wikipedia by expanding it.v · Categories: - Chemical reaction stubs
- Carbon-carbon bond forming reactions
- Condensation reactions
- Quinoline forming reactions
- Multiple component reactions
- Name reactions
Wikimedia Foundation. 2010.
Look at other dictionaries:
Doebner–Miller reaction — The Doebner Miller reaction is the organic reaction of an aniline with α,β unsaturated carbonyl compounds to form quinolines.[1][2][3][4] … Wikipedia
Doebner-Miller reaction — The Doebner Miller reaction is the organic reaction of an aniline with α,β unsaturated carbonyl compounds to form quinolines. [Doebner, O.; Miller, W. v. Ber. 1881, 14 , 2812.] [Doebner, O.; Miller, W. v. Ber. 1883, 16 , 1664 2464.] [Doebner, O.; … Wikipedia
Povarov reaction — [ frame|Scheme 3 Four component Povarov reaction. In order to clarify the role of the lewis acid, a solid scandium nitrogen bond is drawn. Reaction conditions 2 days in acetonitrile at room temperature] The Povarov reaction is a chemical reaction … Wikipedia
Reacción de Doebner-Miller — La reacción de Doebner Miller es una reacción orgánica de una anilina con un compuesto carbonílico α,β insaturado para formar quinolinas.[1] [2] [3] [4] … Wikipedia Español
Skraup reaction — The Skraup synthesis is a chemical reaction used to synthesize quinolines. It is named after the Czech chemist Zdenko Hans Skraup (1850 1910). In the archetypal Skraup, aniline is heated with sulfuric acid, glycerol, and an oxidizing agent to… … Wikipedia
List of organic reactions — Well known reactions and reagents in organic chemistry include Contents: A B C D E F G H I J K L M N O P Q R S T U V W X Y Z See also Ext … Wikipedia
Combes quinoline synthesis — The Combes quinoline synthesis is a chemical reaction involving the condensation of unsubstituted anilines (1) with β diketones (2) to form substituted quinolines (4) after an acid catalyzed ring closure of an intermediate Schiff base (3).[1][2] … Wikipedia
Camps quinoline synthesis — The Camps quinoline synthesis (also known as the Camps cyclization) is a chemical reaction whereby an o acylaminoacetophenone is transformed into two different hydroxyquinolines (products A and B) using hydroxide ion. [Camps, R.; Ber. 1899, 22 ,… … Wikipedia
Conrad-Limpach synthesis — The Conrad Limpach synthesis is the chemical reaction of anilines (1) with β ketoesters (2) to form 4 hydroxyquinolines (4) via a Schiff base (3).[1][2] … Wikipedia
Knoevenagel condensation — The Knoevenagel condensation reaction is an organic reaction named after Emil Knoevenagel. It is a modification of the Aldol condensation[1][2]. A Knoevenagel condensation is a nucleophilic addition of an active hydrogen compound to a carbonyl… … Wikipedia
18+© Academic, 2000-2024- Contact us: Technical Support, Advertising
Dictionaries export, created on PHP, Joomla, Drupal, WordPress, MODx.Share the article and excerpts
Doebner reaction
- Doebner reaction
-
The Doebner reaction is the chemical reaction of an aniline with an aldehyde and pyruvic acid to form quinoline-4-carboxylic acids.[1][2]
References
See also
- Conrad-Limpach reaction
- Doebner-Miller reaction

This chemical reaction article is a stub. You can help Wikipedia by expanding it.