- Methylecgonidine
-
Methylecgonidine 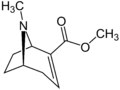 Methyl (1S,5R)-8-methyl-8-azabicyclo[3.2.1]oct-3-ene-4-carboxylateOther namesAnhydromethylecgonine
Methyl (1S,5R)-8-methyl-8-azabicyclo[3.2.1]oct-3-ene-4-carboxylateOther namesAnhydromethylecgonine
Anhydroecgonine methyl esterIdentifiers CAS number 43021-26-7 PubChem 119478 ChemSpider 21106453 
Jmol-3D images Image 1 - CN2[C@@H]/1CC[C@@H]2C\C=C\1C(=O)OC
Properties Molecular formula C10H15NO2 Molar mass 181.232 g/mol  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Methylecgonidine (anhydromethylecgonine; anhydroecgonine methyl ester) is a chemical intermediate derived from ecgonine or cocaine.
Methylecgonidine is a pyrolysis product formed when crack cocaine is smoked, making this substance a useful biomarker to specifically test for use of crack cocaine, as opposed to powder cocaine which does not form methylecgonidine as a metabolite.[1] Methylecgonidine has a relatively short half-life of 18-21 minutes, after which it is metabolised to ecgonidine, meaning that the relative concentrations of the two compounds can be used to estimate how recently crack cocaine has been smoked. Methylecgonidine has been shown to be specifically more harmful to the body than other byproducts of cocaine; for example to the heart,[2] lungs[3] & liver.[4]
It is also used in scientific research for the manufacture of phenyltropane analogues such as Troparil, Dichloropane, Iometopane and CFT. Methylecgonidine could also theoretically be used to produce cocaine and so may be a controlled substance in some countries.
When methylecgonidine is made synthetically for research purposes, it is usually produced by reacting cocaine or ecgonine with hydrochloric acid, yielding ecgonidine (anhydroecgonine), followed by methylation to yield methylecgonidine.[5] Alternatively it can be made in a two step reaction by reacting 2,4,6-cycloheptatriene-7-carboxylic acid with first a mixture of methylamine and sodium hydroxide, followed by reaction with a mixture of methanol and sulfuric acid.[6]
References
- ^ Scheidweiler KB, Plessinger MA, Shojaie J, Wood RW, Kwong TC. Pharmacokinetics and pharmacodynamics of methylecgonidine, a crack cocaine pyrolyzate. Journal of Pharmacology and Experimental Therapeutics. 2003 Dec;307(3):1179-87.
- ^ Pharmacokinetics and Pharmacodynamics of Methylecgonidine, a Crack Cocaine Pyrolyzate - Scheidweiler et al. 307 (3): 1179 Figure IG6 - Journal of Pharmacology And Experimental Therapeutics
- ^ British Journal of Pharmacology - Abstract of article: Evidence for cocaine and methylecgonidine stimulation of M2 muscarinic receptors in cultured human embryonic lung cells
- ^ Studies on Hydrolytic and Oxidative Metabolic Pathways of Anhydroecgonine Methyl Ester (Methylecgonidine) Using Microsomal Preparations from Rat Organs (Chemical Research in Toxicology/ACS Publications)
- ^ Satendra Singh. Chemistry, Design, and Structure-Activity Relationship of Cocaine Antagonists. Chemistry Reviews (2000); (100):925-1024
- ^ Kline RH, Wright J, Fox KM, Eldefrawi ME. Journal of Medicinal Chemistry. (1990); (33):2024
Categories:- Tropanes
- Carboxylic acids
- Alkenes
Wikimedia Foundation. 2010.
